Edgewise Therapeutics Announces Positive Two-Year Topline Results from the ARCH Open Label Trial of Sevasemten (EDG-5506) in Adults with Becker Muscular Dystrophy (Becker)
– The North Star Ambulatory Assessment (NSAA) remained stable relative to declines reported in Becker natural history studies –
– Significant decreases were observed in circulating levels of creatine kinase (CK) and fast skeletal muscle troponin I (TNNI2), biomarkers associated with skeletal muscle damage –
– Sevasemten was well-tolerated –
– Edgewise leadership to discuss ARCH findings on Tuesday, April 16 at 8:30 a.m. Eastern Time at virtual investor event –
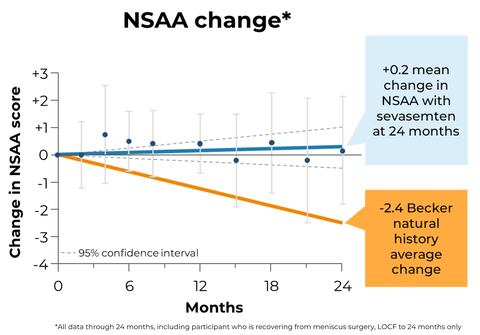
Figure 1: NSAA stabilized with sevasemten treatment and continues to diverge from natural history studies at 2 years (Photo: Business Wire)
The ARCH trial evaluated sevasemten administered daily over two years in adults with Becker. Sevasemten was well-tolerated in all 12 participants with no discontinuations or dose reductions due to adverse events.
Preserving NSAA functions that correlate to activities of daily living are important to individuals living with Becker. As seen in Figure 1, during two years of sevasemten treatment, participants’ NSAA scores stabilized and continued to diverge relative to functional declines reported across multiple Becker natural history studies, in which two-year mean decreases of 2.4 NSAA points were reported1, 2,3.
In addition, significant decreases in key biomarkers of muscle damage including CK and TNNI2 were observed in participants treated with sevasemten, which are consistent with prior observations.
“We are pleased by the promising and consistent functional results observed over two years of treatment with sevasemten, together with the favorable safety and tolerability profile,” said Joanne Donovan, M.D., Ph.D., Chief Medical Officer of Edgewise. “We thank the Becker community for engaging with us on this promising therapy.”
“Becker is a devastating neuromuscular disease that currently has no treatment,” said Barry J. Byrne, M.D., Ph.D., Director, Powell Gene Therapy Center, University of
The positive results from the two-year ARCH trial further support the hypothesis that a reduction in contraction-induced muscle damage in muscular dystrophies has the potential to preserve function and halt disease progression in Becker.
Upcoming ARCH 24-Month Data Presentations with Investor, Medical and Patient Communities:
Virtual Investor Event
Members of the Edgewise management team will hold a live webcast on Tuesday, April 16, at 8:30 a.m. ET to discuss the ARCH two-year data, and will be joined by Dr. Byrne, who will share his perspective of sevasemten and Becker. An accompanying slide presentation will also be available. To register for the live webcast and replay, please visit the Edgewise events page.
American Academy of Neurology 2024 Annual Meeting Podium Presentation
Session: Inherited Myopathies and Neuropathies: New Therapeutic Approaches and Observations
Title: Effects of EDG-5506, a Fast Myosin Modulator, on Function and Biomarkers of Muscle Damage in Adults with Becker Muscular Dystrophy
Presenter: Joanne Donovan, M.D., Ph.D., Chief Medical Officer, Edgewise Therapeutics
Date: Tuesday, April 16, 2024, 1:12 p.m. ET
The presentation will be available on the Edgewise website when it’s presented.
Patient Community Webinar
Members of Edgewise management will hold a community webinar on Monday, May 13 at 1 p.m. ET to discuss these data and the GRAND CANYON pivotal study. Further event details will be shared when they are available.
About the ARCH Open-Label Trial
ARCH, an open-label, single-center trial, assessed sevasemten in 12 adult males with Becker. The trial evaluated sevasemten administered daily over two years. Safety, tolerability, PK, changes in biomarkers of muscle damage such as CK and fast skeletal muscle troponin I, measures of function and patient-reported outcomes were evaluated. Go to clinicaltrials.gov to learn more about this trial (NCT05160415).
About GRAND CANYON, a Global Pivotal Study in Becker
The Company is advancing GRAND CANYON, a global pivotal study of EDG-5506 in individuals with Becker. GRAND CANYON is an expansion of the CANYON placebo-controlled trial. CANYON, which was over-enrolled, includes cohorts of 40 adults and 29 adolescents and a treatment period of 12 months. The Company expects to report CANYON data in the fourth quarter of 2024. GRAND CANYON is a multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of EDG-5506 in adults with Becker. Data from GRAND CANYON, if positive, could support a marketing application. The primary endpoint of GRAND CANYON is NSAA. In addition, other functional assessments, biomarkers of muscle damage and safety will be assessed. GRAND CANYON is anticipated to recruit approximately 120 individuals with Becker, aged between 18 and 50 years old, at up to 50 sites in 10 countries. The treatment period for participants will be 18 months. To learn more, go to clinicaltrials.gov (NCT05291091) or the GRAND CANYON microsite: https://www.beckergcstudy.com.
About Becker Muscular Dystrophy
Becker is a rare, genetic, life-shortening, debilitating and degenerative neuromuscular disorder. The disease predominantly affects males and imposes significant physical, emotional, financial, and social impacts on the individual and their caregivers. Individuals with Becker experience contraction-induced muscle damage, which is the primary driver of muscle loss and impaired motor function in muscular dystrophies. Functional decline can begin at any age, and once that muscle loss occurs, the decline in function is irreversible and continues throughout the individual’s life. Some individuals living with Becker experience heart failure from cardiomyopathy, which may result in heart transplantation or early death. Currently, there is no cure for Becker; early and long-term multidisciplinary care is critical for optimized disease management. There is a great need for more Becker-specific scientific research, clinical programs, and treatment guidelines to improve management of this disease. To learn more about Becker, go to https://beckermusculardystrophy.com/
About Sevasemten (EDG-5506) for Becker and Duchenne Muscular Dystrophies
Sevasemten is an orally administered small molecule designed to prevent contraction-induced muscle damage in dystrophinopathies including Becker and Duchenne. Sevasemten presents a novel mechanism of action designed to selectively limit the exaggerated muscle damage caused by the absence or loss of functional dystrophin. By minimizing the progressive muscle damage that leads to functional impairment, sevasemten has the potential to benefit a broad range of patients suffering from debilitating neuromuscular disorders. Its unique mechanism of action provides the potential to establish sevasemten as a foundational therapy in dystrophinopathies, either as a single agent therapy or in combination with available therapies and those in development.
In Becker, Edgewise is advancing sevasemten in a Phase 2 trial, called CANYON, evaluating safety and effects on function and biomarkers of muscle damage in adult males with Becker. The CANYON trial, which is fully enrolled, has been expanded to include an additional 120 adult participants in a pivotal cohort called GRAND CANYON. This study is currently enrolling at sites in
In Duchenne, Edgewise is advancing its LYNX Phase 2 clinical trial, assessing safety, PK, biomarkers of muscle damage and functional measures in boys with Duchenne. It also is advancing a second Phase 2 trial, called FOX, assessing safety, PK, biomarkers of muscle damage and functional measures in children and adolescents previously treated with gene therapy.
For more information on Edgewise’s clinical trials https://edgewisetx.com/clinical-trials.
About Edgewise Therapeutics
Edgewise Therapeutics is a leading muscle disease biopharmaceutical company developing novel therapeutics for muscular dystrophies and serious cardiac conditions. The Company’s deep expertise in muscle physiology is driving a new generation of first-in-class therapeutics. Sevasemten is an orally administered skeletal myosin inhibitor in late-stage clinical trials in Becker and Duchenne muscular dystrophies. EDG-7500 is a novel cardiac sarcomere modulator for the treatment of hypertrophic cardiomyopathy and other diseases of diastolic dysfunction, currently in clinical development. The entire team at Edgewise is dedicated to our mission: changing the lives of patients and families affected by serious muscle diseases. To learn more, go to: www.edgewisetx.com or follow us on LinkedIn, X (formerly Twitter), Facebook, Instagram and Threads.
References
[1] Bello L, et al. Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies. Sci Rep. 2016;6:32439. doi:10.1038/srep32439
[2] van de Velde NM, et al. Selection approach to identify the optimal biomarker using quantitative muscle MRI and functional assessments in Becker muscular dystrophy. Neurology. 2021;97(5):e513-e522. doi: 10.1212/WNL.0000000000012233.
[3] De Wel B, et al. Lessons for future clinical trials in adults with Becker muscular dystrophy: disease progression detected by muscle magnetic resonance imaging, clinical and patient-reported outcome measures. Eur J Neurol. 2024:e16282. doi:10.1111/ene.16282. Online ahead of print.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential of, and expectations regarding Edgewise’s expectations relating to its clinical trials and clinical development of sevasemten; statements regarding the potential of, and expectations regarding, Edgewise’s product candidates and programs, including EDG-5506 and EDG-7500; statements regarding Edgewise’s milestones, including timing of data from its CANYON trial; statements regarding whether data from GRAND CANYON could support a marketing application; and statements by Edgewise’s chief medical officer and Barry J. Byrne, M.D., Ph.D.. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon Edgewise’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics and operating as an early clinical stage company including the potential for Edgewise’s product candidates to cause serious adverse events; Edgewise’s ability to develop, initiate or complete clinical trials for, obtain approvals for and commercialize any of its product candidates; Edgewise’s ability to take advantage of potential benefits associated with designations granted by FDA and/or to maintain qualifications for applicable designations over time; the timing, progress and results of clinical trials for EDG-5506 and EDG-7500; Edgewise’s ability to enroll and maintain patients in clinical trials; Edgewise’s ability to raise any additional funding it will need to continue to pursue its business and product development plans; the timing, scope and likelihood of regulatory filings and approvals; the potential for any clinical trial results to differ from preclinical, interim, preliminary, topline or expected results; the potential that the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials; Edgewise’s ability to develop a proprietary drug discovery platform to build a pipeline of product candidates; Edgewise’s manufacturing, commercialization and marketing capabilities and strategy; the size of the market opportunity for Edgewise’s product candidates; the loss of key scientific or management personnel; competition in the industry in which Edgewise operates; Edgewise’s reliance on third parties; Edgewise’s ability to obtain and maintain intellectual property protection for its product candidates; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that Edgewise files from time to time with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240415221564/en/
Edgewise Contacts
Investors:
Michael Carruthers, Chief Financial Officer
ir@edgewisetx.com
Media:
Maureen Franco, VP Corporate Communications
media@edgewisetx.com
Source: Edgewise Therapeutics






