FDA Approves Biohaven's NURTEC® ODT (rimegepant) for Prevention: Now the First and Only Migraine Medication for both Acute and Preventive Treatment
Biohaven Pharmaceutical announced the FDA approval of NURTEC® ODT (rimegepant 75 mg) for the preventive treatment of migraine, marking it as the first oral CGRP antagonist to receive such approval. This new label allows for up to 18 doses per month, offering both acute and preventive therapy. The approval significantly expands the market potential for NURTEC ODT, benefiting the approximately 95% of U.S. migraine patients with fewer than 15 headache days monthly. The medication's novel formulation provides flexible treatment options, covering a substantial unmet need in migraine care.
- FDA approved NURTEC ODT for preventive migraine treatment, expanding market potential.
- NURTEC ODT is the first oral medication for both acute and preventive migraine treatment.
- Clinical trial showed a significant reduction in monthly migraine days by 4.3 days after three months.
- NURTEC ODT contains contraindications for patients with hypersensitivity to its components.
Insights
Analyzing...
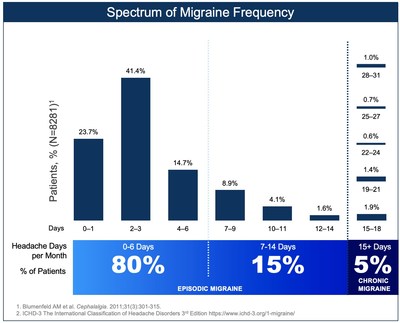
NEW HAVEN, Conn., May 27, 2021 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), today announced that the U.S. Food and Drug Administration (FDA) has approved NURTEC® ODT (rimegepant 75 mg) for the preventive treatment of migraine. NURTEC® ODT is indicated for adult patients with episodic migraine, e.g. those who experience less than 15 headache days per month. The approved product label was also expanded to include the use of NURTEC ODT 75 mg up to 18 doses/month, allowing for both acute and preventive therapy in the same patient. This new approval makes NURTEC ODT the first oral CGRP antagonist approved for the preventive treatment of migraine, and the only migraine medication approved as a dual therapy for both the acute and preventive treatment. NURTEC ODT is approved for acute treatment in all eligible adult patients with migraine, regardless of the number of monthly migraine days. Since approximately
Peter J. Goadsby, M.D., Ph.D., D.Sc., Professor of Neurology at the University of California, Los Angeles and King's College London, recipient of the 2021 Brain Prize for his groundbreaking research discovering the role of CGRP in migraine, and co-author of the Phase 3 preventive study published in The Lancet, commented, "The FDA approval of NURTEC ODT for the preventive treatment of migraine--along with its acute treatment indication--is one of the most groundbreaking things to happen to migraine treatment in my 40 years of practicing headache medicine. To have one medication patients can use to treat and prevent migraine will likely change the treatment paradigm for many of the millions of people who live with migraine."
NURTEC ODT, with its novel quick-dissolve tablet formulation, works by blocking the CGRP receptor, treating a root cause of migraine. With this targeted mechanism of action, NURTEC ODT provides a more complete, flexible treatment plan that gives people with migraine increased control of their disease. A single dose of NURTEC ODT can deliver fast pain relief that lasts up to 48 hours for many patients. NURTEC ODT can be taken up to once daily as needed to stop migraine attacks or taken every other day to help prevent migraine and reduce the number of monthly migraine days. This approval represents an important advancement in care for the several hundreds of millions of people living with migraine across the globe. For the first time, one medication can treat and prevent migraine attacks.
Vlad Coric, M.D., Chief Executive Officer of Biohaven noted, "This FDA approval marks the beginning of a new era for migraine treatments, allowing the potential for healthcare professionals to prescribe, and patients to have, a single medication to treat and prevent migraine attacks. NURTEC ODT is dissolving the line between acute and preventive treatment. This groundbreaking approach to treating the full spectrum of migraine disease, from acute therapy to prevention, can have a significant impact in a patient's life by helping to decrease treatment plan complexity and reduce challenges with adherence and polypharmacy. At Biohaven, neuroinnovation is at the center of our focus and we are committed to finding and advancing paradigm shifting treatment options to help people with neurological diseases so they can live life without the burden of these illnesses."
The FDA approval of NURTEC ODT is based on a double-blind, randomized, placebo-controlled Phase 3 clinical trial with an open label extension. Primary study endpoint results demonstrated that NURTEC was superior to placebo, decreasing monthly migraine days by 4.3 days/month after three months of treatment. The preventive effects of NURTEC were seen as early as the first week of therapy. Further, a key secondary endpoint result showed that approximately half of NURTEC-treated patients had a
Carolyn Armitage, a NURTEC preventive clinical trial participant shared, "As a middle and high school teacher, I know how important it is to show up every day for my students. I value the opportunity my career provides me to help shape young minds and lives. But having a migraine attack almost three times a week has had a devastating impact on my professional and personal life. I wasn't able to be present for my students, family or friends. After trying NURTEC ODT through the clinical trial, I'm so pleased that the frequency and severity of my migraine have subsided making them more manageable."
In a pivotal trial for the preventive treatment of migraine, NURTEC was generally well tolerated with the most common side effects being nausea (
A recent survey, Preventing Migraine Attacks: A Current Perspective by the National Headache Foundation confirms that 84 percent of people currently taking a preventive treatment wish there was a better treatment option. Among respondents,
Biohaven Patient Access Commitment
NURTEC ODT is now available in pharmacies for the acute and preventive treatment of migraine. People with migraine should talk with their healthcare provider about using NURTEC ODT to treat and prevent migraine attacks.
Biohaven is committed to supporting the migraine community by eliminating barriers to medication access. The company plans to continue the
About NURTEC ODT
NURTEC® ODT (rimegepant) is the first and only calcitonin gene-related peptide (CGRP) receptor antagonist available in a quick-dissolve ODT formulation that is approved by the U.S. Food and Drug Administration (FDA) for the acute treatment of migraine with or without aura and the preventive treatment of episodic migraine in adults. The activity of the neuropeptide CGRP is thought to play a causal role in migraine pathophysiology. NURTEC ODT is a CGRP receptor antagonist that works by reversibly blocking CGRP receptors, thereby inhibiting the biologic activity of the CGRP neuropeptide. The recommended dose of NURTEC ODT is 75 mg, taken as needed, up to once daily to treat or every other day to help prevent migraine attacks. For more information about NURTEC ODT, visit www.NURTEC.com. The most common adverse reaction was nausea and abdominal pain/indigestion. Avoid concomitant administration of NURTEC ODT with strong inhibitors of CYP3A4, strong or moderate inducers of CYP3A or inhibitors of P-gp or BCRP. Avoid another dose of NURTEC ODT within 48 hours when it is administered with moderate inhibitors of CYP3A4.
Indication
NURTEC ODT orally disintegrating tablets is a prescription medicine that is used to treat migraine in adults. It is for the acute treatment of migraine attacks with or without aura and the preventive treatment of episodic migraine. It is not known if NURTEC ODT is safe and effective in children.
Important Safety Information
Do not take NURTEC ODT if you are allergic to NURTEC ODT (rimegepant) or any of its ingredients.
Before you take NURTEC ODT, tell your healthcare provider (HCP) about all your medical conditions, including if you:
- have liver problems,
- have kidney problems,
- are pregnant or plan to become pregnant,
- breastfeeding or plan to breastfeed.
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
NURTEC ODT may cause serious side effects including allergic reactions, including trouble breathing and rash. This can happen days after you take NURTEC ODT. Call your HCP or get emergency help right away if you have swelling of the face, mouth, tongue, or throat or trouble breathing. This occurred in less than
The most common side effects of NURTEC ODT were nausea (
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1–800–FDA–1088 or report side effects to Biohaven at 1–833–4NURTEC.
See full Prescribing Information and Patient Information.
About Migraine
Migraine is the 3rd most prevalent illness in the world and the World Health Organization classifies migraine as one of the 10 most disabling medical illnesses. Approximately 40 million people in the U.S., and hundreds of millions worldwide, suffer from migraine. Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity that can be associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia). There is a significant unmet need for new acute and preventive treatments as more than 90 percent of people with migraine are unable to work or function normally during an attack.
About CGRP Receptor Antagonism
Small molecule CGRP receptor antagonists represent a novel class of drugs for the treatment of migraine. This unique mode of action potentially offers an alternative to current agents, particularly for patients who have contraindications to the use of triptans, or who have a poor response to triptans or are intolerant to them.
About Biohaven
Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's neuroinnovation portfolio includes FDA-approved NURTEC ODT (rimegepant) for the acute and preventive treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and MPO inhibition for multiple system atrophy and amyotrophic lateral sclerosis. More information about Biohaven is available at www.biohavenpharma.com.
Forward-looking Statement
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of Biohaven's management. All statements, other than statements of historical facts, included in this press release regarding Biohaven's business and product candidate plans and objectives are forward-looking statements. Forward-looking statements include those related to: the preliminary nature of net product revenues for NURTEC ODT, commercialization and sales of NURTEC ODT and the potential approval and commercialization of other product candidates, the effect of the ongoing COVID-19 pandemic on Biohaven, the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials, the timing of planned interactions and filings with the FDA, the timing and outcome of expected regulatory filings, including the need for any REMS or Advisory Committee meetings, the potential for Biohaven's product candidates to be first in class or best in class therapies and the effectiveness and safety of Biohaven's product candidates. The use of certain words, including "believe", "continue", "may", "on track", "expects" and "will" and similar expressions, are intended to identify forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2020, filed with the Securities and Exchange Commission on March 1, 2021, and Biohaven's Quarterly Report on Form 10-Q for the quarter ended March 31, 2021, filed with the Securities and Exchange Commission on May 10, 2021. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC.
Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
Biohaven Contact
Dr. Vlad Coric
Chief Executive Officer
Vlad.Coric@biohavenpharma.com
Media Contact
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-approves-biohavens-nurtec-odt-rimegepant-for-prevention-now-the-first-and-only-migraine-medication-for-both-acute-and-preventive-treatment-301301304.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/fda-approves-biohavens-nurtec-odt-rimegepant-for-prevention-now-the-first-and-only-migraine-medication-for-both-acute-and-preventive-treatment-301301304.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.