Biohaven Highlights Portfolio Progress, Innovation, and Anticipated Milestones at the 43rd Annual J.P. Morgan Healthcare Conference; Reports Positive Degrader Data with Rapid, Deep, and Selective Lowering of Galactose-Deficient IgA1 with Next Generation Potential Therapy for IgA Nephropathy
Biohaven highlighted significant progress across its portfolio at the 43rd Annual J.P. Morgan Healthcare Conference, featuring positive Phase 1 data for BHV-1400, its IgA nephropathy treatment. The first and lowest dose (125mg) achieved rapid lowering of Gd-IgA1 with a 60% median reduction within four hours and over 70% reduction within eight hours.
Key developments include:
- Advancement of MoDE platform and TRAP degraders across multiple programs
- Progress in ion channel programs for bipolar disorder, depression, epilepsy, and pain
- Expansion of antibody drug conjugate portfolio in oncology
- Development of BHV-1300 for Graves' Disease showing >60% IgG reduction
- Planned pivotal trials for BHV-8000 in Parkinson's and Alzheimer's disease in 2025
- NDA submission for troriluzole in SCA genotypes
Biohaven ha messo in evidenza progressi significativi nel suo portafoglio durante la 43ª Conferenza Annuale J.P. Morgan Healthcare, presentando dati positivi della Fase 1 per BHV-1400, il suo trattamento per la nefropatia da IgA. La prima e più bassa dose (125 mg) ha ottenuto una rapida riduzione di Gd-IgA1 con una riduzione mediana del 60% entro quattro ore e oltre il 70% entro otto ore.
Sviluppi chiave includono:
- Avanzamento della piattaforma MoDE e degradatori TRAP attraverso diversi programmi
- Progresso nei programmi sui canali ionici per il disturbo bipolare, la depressione, l'epilessia e il dolore
- Espansione del portafoglio di coniugati anticorpali in oncologia
- Sviluppo di BHV-1300 per la malattia di Graves che mostra una riduzione >60% di IgG
- Prove pivotal planned per BHV-8000 nella malattia di Parkinson e Alzheimer nel 2025
- Invio della NDA per troriluzole nelle genotipizzazioni SCA
Biohaven destacó avances significativos en su portafolio en la 43ª Conferencia Anual de Atención Médica J.P. Morgan, presentando datos positivos de Fase 1 para BHV-1400, su tratamiento para la nefropatía por IgA. La primera y más baja dosis (125 mg) logró una rápida reducción de Gd-IgA1 con una reducción media del 60% en cuatro horas y más del 70% en ocho horas.
Los desarrollos clave incluyen:
- Avance de la plataforma MoDE y degradadores TRAP en múltiples programas
- Progreso en programas de canales iónicos para trastorno bipolar, depresión, epilepsia y dolor
- Expansión de la cartera de conjugados de medicamentos anticuerpos en oncología
- Desarrollo de BHV-1300 para la enfermedad de Graves mostrando >60% de reducción de IgG
- Pruebas fundamentales planificadas para BHV-8000 en la enfermedad de Parkinson y Alzheimer en 2025
- Presentación de NDA para troriluzole en genotipos de SCA
Biohaven은 43회 J.P. Morgan Healthcare Conference에서 자사 포트폴리오의 중요한 진행 상황을 강조하며, BHV-1400, IgA 신병 치료에 대한 긍정적인 1상 데이터를 발표했습니다. 첫 번째 및 최저 용량인 125mg은 4시간 이내에 Gd-IgA1을 60% 중간 감소시키고 8시간 이내에 70% 이상 감소시키는 빠른 효과를 보였습니다.
주요 개발 사항은 다음과 같습니다:
- 여러 프로그램에서 MoDE 플랫폼 및 TRAP 분해제의 진전
- 양극성 장애, 우울증, 간질, 통증을 위한 이온 채널 프로그램의 진전
- 온콜로지에서 항체 약물 접합체 포트폴리오의 확장
- 그레이브병에 대한 BHV-1300 개발, IgG가 >60% 감소함
- 2025년 파킨슨병 및 알츠하이머병을 위한 BHV-8000의 주요 시험 계획
- SCA 유전자형에 대한 troriluzole의 NDA 제출
Biohaven a souligné des progrès significatifs dans son portefeuille lors de la 43e Conférence Annuelle J.P. Morgan Healthcare, présentant des données positives de Phase 1 pour BHV-1400, son traitement pour la néphropathie à IgA. La première et plus basse dose (125 mg) a permis une réduction rapide de Gd-IgA1 avec une réduction médiane de 60 % en quatre heures et plus de 70 % en huit heures.
Les développements clés comprennent :
- Avancement de la plateforme MoDE et des dégradateurs TRAP à travers plusieurs programmes
- Progrès dans les programmes de canaux ioniques pour le trouble bipolaire, la dépression, l'épilepsie et la douleur
- Expansion du portefeuille de conjugués médicament-anticorps en oncologie
- Développement de BHV-1300 pour la maladie de Basedow montrant une réduction >60% d'IgG
- Essais pivots prévus pour BHV-8000 dans la maladie de Parkinson et d'Alzheimer en 2025
- Soumission de la NDA pour le troriluzole dans les génotypes SCA
Biohaven hob bedeutende Fortschritte in seinem Portfolio auf der 43. jährlichen J.P. Morgan Healthcare Conference hervor, bei der positive Phase-1-Daten für BHV-1400, seine Behandlung für IgA-Nephropathie, präsentiert wurden. Die erste und niedrigste Dosis (125 mg) erzielte innerhalb von vier Stunden eine schnelle Senkung von Gd-IgA1 mit einer medianen Reduktion von 60 % und über 70 % Reduktion innerhalb von acht Stunden.
Wichtige Entwicklungen umfassen:
- Fortschritt der MoDE-Plattform und TRAP-Degrader über mehrere Programme hinweg
- Fortschritte bei Ionenkanalprogrammen für bipolare Störungen, Depressionen, Epilepsie und Schmerzen
- Erweiterung des Portfolios von Antikörper-Wirkstoff-Konjugaten in der Onkologie
- Entwicklung von BHV-1300 für die Basedow-Krankheit mit einer Reduktion von >60 % IgG
- Geplante wichtige Studien für BHV-8000 bei Parkinson und Alzheimer im Jahr 2025
- NDA-Einreichung für troriluzole bei SCA-Genotypen
- BHV-1400 achieved unprecedented 60% reduction in Gd-IgA1 within 4 hours and 70% within 8 hours in Phase 1
- BHV-1300 demonstrated >60% IgG reduction in lowest subcutaneous dose tested
- FDA alignment received for accelerated approval pathway for BHV-1600 in PPCM
- Multiple INDs accepted by FDA in 2024 for MoDE and TRAP degrader molecules
- NDA submitted for troriluzole with potential Priority Review
- None.
Insights
The data from Biohaven's MoDE and TRAP degrader platforms represents a groundbreaking advancement in precision immunology. The key highlight is BHV-1400's unprecedented achievement in IgA nephropathy - showing 60% reduction in Gd-IgA1 within 4 hours while preserving normal immune function. This selective targeting capability could revolutionize treatment approaches across multiple autoimmune conditions.
The company's diversified pipeline spanning 10+ assets demonstrates strong execution potential. The BHV-1300 program's progress in Graves' Disease, targeting a $80+ billion global market opportunity, shows particular promise with its optimized subcutaneous delivery format and robust efficacy data.
From a commercial perspective, Biohaven's expanded collaboration with GeneQuantum for ADC development and new partnership with Merus significantly strengthens their oncology portfolio. These strategic moves help de-risk the pipeline while maintaining strong upside potential.
The rapid and selective protein degradation achieved by BHV-1400 represents a paradigm shift in treating autoimmune diseases. The ability to specifically target pathogenic proteins while sparing normal immune function addresses a critical limitation of current broad-spectrum immunosuppressive approaches.
The safety profile is particularly impressive - showing no significant impacts on white blood cells, immunoglobulins, or liver function. This selective targeting could potentially eliminate many side effects associated with existing treatments like complement inhibitors or B-cell depleting therapies.
The accelerated regulatory pathway discussions with FDA for both BHV-1400 and BHV-1600 suggest confidence in the platform's potential. The 70% maximal reduction in Gd-IgA1 within 8 hours could enable novel acute treatment paradigms beyond chronic disease management.
The breadth of Biohaven's pipeline provides multiple shots on goal across high-value indications. The Graves' Disease program alone targets 80 million patients globally, while rare disease opportunities like peripartum cardiomyopathy address critical unmet needs.
The platform's versatility is evidenced by next-generation programs targeting IgG4, PLA2R, pro-insulin and TSH receptor autoantibodies. This suggests significant potential for pipeline expansion and validates the technology's broad applicability.
The strategic focus on patient-friendly subcutaneous formulations and autoinjector development with Ypsomed demonstrates strong commercial foresight. Multiple potential catalysts in 2025, including pivotal trial initiations and Phase 1 completions, could drive significant value creation.
- Presents progress and new anticipated milestones across portfolio of more than 10 assets and 6 therapeutic areas.
- Announces multiple advancements across the MoDETM (molecular degraders of extracellular proteins) platform and the next generation TRAPTM (targeted removal of aberrant protein) degraders, including:
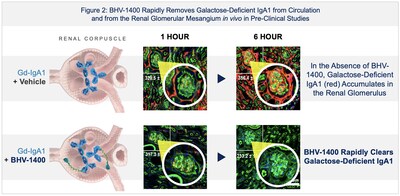
- IgA Nephropathy (IgAN) program: First-in-human dosing with BHV-1400, a next generation TRAP degrader, achieved rapid, deep, and selective lowering of only aberrant galactose-deficient IgA1 (Gd-IgA1), the antibody causing IgA nephropathy, while sparing normal IgA.
- This selective and rapid approach to immunoglobulin lowering represents a second-generation therapeutic approach to IgAN, potentially allowing for strong disease control with less acute or long-term safety risks associated with complement inhibition or broad antibody suppression.
- The first and lowest dose tested (125mg) of BHV-1400 in the ongoing Phase 1 achieved rapid lowering of Gd-IgA1 with a median reduction of
60% within four hours of administration after a single dose. Maximal reduction exceeding70% was observed within eight hours. Reductions were sustained for days even after a single dose. This rapid reduction of Gd-IgA1 is unprecedented in drugs targeting Gd-IgA1 and could allow for potential indications in situations where rapid Gd-IgA1 lowering could be beneficial in addition to chronic active disease and long-term maintenance. - BHV-1400 has been safe and well-tolerated in the Phase 1 study to date and demonstrated no clinically significant changes in innate or adaptive immunity, including white blood cells and immunoglobulins IgG, IgA, IgE, and IgM. There were no clinically significant reductions in albumin, liver function test abnormalities, or increases in cholesterol compared to baseline.
- No dose limiting toxicities have been observed in the Phase 1 to date and further dose escalation is planned to explore the full range of Gd-IgA1 reductions possible with BHV-1400.
- A pivotal trial in IgA nephropathy is planned using an accelerated regulatory path forward upon completion of the Phase 1 trial.
- Peripartum cardiomyopathy (PPCM) program: First-in-human dosing with BHV-1600, a TRAP degrader of β1AR autoantibodies, was initiated and has been well-tolerated to date after the first two dose cohorts without clinically relevant changes in innate or adaptive immunity, including white blood cells and immunoglobulins IgG, IgA, IgE, and IgM. There were no clinically significant reductions in albumin, liver function test abnormalities, or increases in cholesterol compared to baseline. β1AR autoantibodies are thought to cause peripartum cardiomyopathy, a rare form of heart failure that develops at the end of pregnancy or in the months following delivery and in severe cases, can be life-threatening. BHV-1600 has been shown to bind to β1AR autoantibodies in preclinical studies and biomarker levels will be measured in women with PPCM later in development.
- Completed INTERACT meeting with FDA regarding BHV-1600 in 4Q 2024; gained alignment for the study design to potentially pursue an accelerated approval pathway in PPCM.
- IgG degrader programs:
- BHV-1300: Advanced optimized subcutaneous formulation with deep reductions of targeted IgG >
60% demonstrated in the lowest dose cohort of the MAD, in line with modeling projections and with differentiated safety profile. There were no clinically significant reductions in IgG3, IgA, IgE, IgM, or albumin, no liver function test abnormalities, nor increases in cholesterol compared to baseline. - Phase 1 completing last remaining dose cohorts with the optimized subcutaneous formulation in 1H 2025.
- Lead indication for BHV-1300 announced in Graves' Disease, a common autoimmune disorder affecting approximately 80 million people globally. Graves' Disease is caused by IgG1 autoantibodies that hyperstimulate the TSH receptor, causing hyperthyroidism can result in the need for surgical removal, chemical ablation of the thyroid, or need for chronic anti-thyroid drugs. Additional programs in rheumatoid arthritis and myasthenia gravis also continue to be pursued with BHV-1300 and 1310.
- Study May Proceed letter received from FDA for BHV-1310 IND, a novel IgG degrader and Phase 1 initiating in 1H 2025. BHV-1300 and BHV-1310 are similar but will optimize therapeutic targeting and facilitate broader commercial development options.
- BHV-1300: Advanced optimized subcutaneous formulation with deep reductions of targeted IgG >
- Next generation degrader targets advancing in 2025 include: IgG4 specific degrader, PLA2R autoantibody degrader for membranous nephropathy, pro-insulin autoantibody degrader for type 1 diabetes, and TSH receptor autoantibody degrader as a selective follow-on asset for Graves' Disease.
- IgA Nephropathy (IgAN) program: First-in-human dosing with BHV-1400, a next generation TRAP degrader, achieved rapid, deep, and selective lowering of only aberrant galactose-deficient IgA1 (Gd-IgA1), the antibody causing IgA nephropathy, while sparing normal IgA.
- Progresses ion channel programs for bipolar, depression, epilepsy and pain:
- BHV-7000, a selective activator of Kv7.2/7.3 potassium channels: Continues to advance in clinical trials for bipolar disorder, depression, and epilepsy with multiple readouts over the next year, including expected topline for bipolar in 1H 2025 and depression in 2H 2025.
- BHV-2100, a TRPM3 ion channel antagonist: Preliminary data from an innovative laser-evoked hyperalgesia study with BHV-2100 reduced laser heat-induced pain and brain evoked potentials in healthy volunteers. These exciting results with BHV-2100 represent the first clinical proof of concept for the novel TRPM3 inhibitor class in pain, recapitulating powerful preclinical evidence of efficacy for this approach across a spectrum of pain disorders.
- Highlights expansion of Biohaven's antibody drug conjugate (ADC) portfolio in Oncology, including:
- BHV-1510, a Trop2-directed ADC using a novel topoisomerase 1 (TopoIx) payload: Demonstration of early Phase 1 clinical activity, including tumor shrinkage, and safety with BHV-1510 in advanced or metastatic epithelial tumors.
- Based upon these results, Biohaven has entered into an expanded collaboration agreement with GeneQuantum providing exclusivity to the TopoIx payload for up to 18 ADC targets.
- BHV-1530, a FGFR3-directed ADC using the TopoIx payload: US IND clearance received for BHV-1530, potential indications include urothelial cancers and other solid tumors driven by FGFR3 alterations and/or upregulated FGFR3 protein expression. The first-in-human study is planned to commence in 1H 2025.
- Announced a multi-target collaboration with Merus, an oncology-focused biotechnology company developing innovative, multi-specific (Biclonics® and Triclonics®) antibodies, to co-develop three programs encompassing highly differentiated next generation dual-targeted bispecific ADCs leveraging Biohaven's proprietary conjugation and payload technologies and Merus' leading Biclonics technology platform.
- BHV-1510, a Trop2-directed ADC using a novel topoisomerase 1 (TopoIx) payload: Demonstration of early Phase 1 clinical activity, including tumor shrinkage, and safety with BHV-1510 in advanced or metastatic epithelial tumors.
- Key late-stage programs advancing across important indications in 2025 also include:
- BHV-8000, a highly selective, brain-penetrant TYK2/JAK1 inhibitor that avoids class risks associated with JAK2/3 inhibition, expected to initiate pivotal trials in Parkinson's and Alzheimer's disease in 2025.
- Taldefgrobep alfa, a novel myostatin inhibitor optimized to block two key regulators of muscle and fat metabolism, continues to be developed in spinal muscular atrophy and obesity.
- New Drug Application (NDA) for troriluzole in all SCA genotypes was submitted to the FDA, following completion of pre-NDA meeting in 4Q 2024. Troriluzole has Orphan Drug and Fast-Track designations and qualifies for potential Priority Review. Awaiting filing decision from FDA and preparing for potential commercial launch in 2025 if approved.
Vlad Coric, M.D., Chairman and Chief Executive Officer of Biohaven, commented, "While we are excited about the significant progress across our entire portfolio, the first-ever data of a TRAP degrader in humans is monumental and unparalleled. The ability to only remove aberrant proteins causing disease while leaving all other immune functioning intact will usher in a new era of precision immunology. As quickly as science can identify new disease-causing proteins, our technology can quickly advance treatments for patients. I am proud of our dedicated, passionate and gifted team's unrelenting drive to transform medical care for patients suffering from severe diseases."
Biohaven 2025 Portfolio Review and Anticipated Milestones
Biohaven is positioned to achieve significant milestones in 2025 across a broad spectrum of early- and late-stage programs targeting indications with high unmet need:
Molecular Degrader of Extracellular Proteins (MoDE) Platform: Biohaven's novel immune-modulating extracellular degrader platform harnesses selectivity, rapidity, and patient-friendly self-administration to remove disease-causing proteins from the body to potentially treat a wide range of diseases. Biohaven introduced next generation TRAP degraders, which are highly selective, each targeting a specific disease-causing protein for proteolysis. Four INDs for MoDE and next generation TRAP degrader molecules (targeting IgG1, IgG2, IgG4, Gd-IgA1, and β1AR autoantibodies) have been accepted by the FDA in 2024 with several additional investigational agents in development. Three assets have been dosed in Phase 1 trials with the fourth anticipated to be dosed in the first half of 2025.
The first-of-its-kind molecule, BHV-1300 is being developed for the treatment of common immune-mediated diseases, such as Graves' Disease and Rheumatoid Arthritis. With patient-centered design, Biohaven has advanced a proprietary subcutaneous formulation of BHV-1300 and has entered into an agreement with Ypsomed, to develop and manufacture BHV-1300 in an easy-to-use, autoinjector for self-administration. This BHV-1300 proprietary formulation has demonstrated deep and targeted reductions of IgG, with reductions >
BHV-1400 and BHV-1600, currently in Phase 1 clinical trials, represent the next generation TRAP degraders focused on selectively clearing very specific pathogenic antibodies, while sparing healthy immunoglobulin to preserve immune function. TRAP molecules commence a new age of immune-modulating treatments, targeted removal of disease-causing proteins for removal while sparing the normal function of the healthy immune system. Data from the first, and lowest, dose cohort of BHV-1400 demonstrated clear differentiation from competitors in the IgA nephropathy space, with rapid lowering of Gd-IgA1 within four hours and preservation of host immunoglobulins including IgG, IgA, IgE, and IgM. IgA nephropathy is a rare chronic kidney disease affecting young and middle-aged adults that is caused by Gd-IgA1 and leads to kidney failure in up to
Taken in total, the selectivity of MoDE and TRAP degraders demonstrated to date refine immune-modulating treatment representing a clear next generation of drug development technology in immunoglobulin and extracellular protein lowering. Existing mechanisms, both pharmaceutical and device (plasmapheresis), broadly reduce immunoglobulins subclasses and/or isotypes, leading to inefficient dosing, safety risks, necessity of procedures, delays in therapy, and potential efficacy impacts. MoDE and TRAP's new paradigm builds off the prior successes of immune modulation, while also providing a novel technology to fine tune therapies for immune-mediated diseases. As described below, the implications and applications of this selective targeting could be multi-organ, multi-disease.
- IgA Nephropathy (IgAN) Program: First-in-human dosing with BHV-1400 achieved rapid, deep, and selective lowering of only Gd-IgA1, the aberrant antibody that causes IgA nephropathy, while sparing normal IgA.
- The first and lowest dose tested (125 mg) of BHV-1400 in Phase 1 achieved rapid lowering of Gd-IgA1 with a median reduction of
60% within four hours of administration. Maximal reduction exceeding70% was observed within eight hours. Even after just a single dose administration of BHV-1400, reductions in Gd-IgA1 were sustained for days. The rapid reduction of Gd-IgA1 by BHV-1400 is unprecedented in drugs targeting Gd-IgA1 and could allow for potential indications in situations where rapid Gd-IgA1 lowering could be beneficial in addition to chronic active disease and long-term maintenance. The selective and rapid approach to Gd-IgA1 lowering of BHV-1400 represents a second-generation therapeutic approach to IgAN, potentially allowing for effective disease control with less acute- or long-term safety risks associated with B-cell directed therapy, complement inhibitors, or broad immunosuppression. - BHV-1400 has been safe and well-tolerated in the Phase 1 study to date and demonstrated no clinically significant changes in innate or adaptive immune systems, including white blood cells and immunoglobulins IgG, IgA, IgE, and IgM, and no clinically significant reductions in albumin, liver function test abnormalities, or increases in cholesterol compared to baseline. There have been no dose limiting toxicities observed in the Phase 1 study and dose escalation continues to explore the full range of Gd-IgA1 reductions possible with BHV-1400.
- A pivotal trial in IgA nephropathy is planned using an accelerated regulatory path upon completion of the Phase 1 trial. Additional opportunities in situations when rapid Gd-IgA1 reduction could be beneficial may also be feasible given the demonstrated dosing kinetics in the Phase 1 study.
- Biohaven further expands its renal franchise with the degrader platform, developing several investigational TRAPs for the treatment of immune-mediated renal disease, including a TRAP degrader to target PLA2R autoantibodies for the treatment of membranous nephropathy among others.
- The first and lowest dose tested (125 mg) of BHV-1400 in Phase 1 achieved rapid lowering of Gd-IgA1 with a median reduction of
- Peripartum cardiomyopathy (PPCM) program: First-in-human dosing with BHV-1600 has been safe and well-tolerated to date after two dose cohorts without clinically relevant changes in innate or adaptive immune systems, including white blood cells and immunoglobulins IgG, IgA, IgE, and IgM, and no clinically significant reductions in albumin, liver function test abnormalities, or increases in cholesterol compared to baseline. β1AR autoantibodies are thought to cause peripartum cardiomyopathy, a rare form of heart failure with no approved therapy that occurs at the end of pregnancy or following delivery and in severe cases, can be life-threatening. BHV-1600 has been shown to bind to β1AR autoantibodies in preclinical studies and biomarker levels will be measured in women with PPCM later in development.
- Completed INTERACT meeting with FDA regarding BHV-1600 in 4Q 2024 and gained alignment for the study design to potentially pursue an accelerated approval pathway in PPCM, a rare autoimmune life-threatening disease with no approved therapy.
- IgG MoDE degrader program: BHV-1300 Phase 1 is completing the last remaining dose cohorts with expected completion in 1H 2025. Study May Proceed letter received from the FDA for the BHV-1310 IND and Phase 1 initiating in 1H 2025.
- Lead indication for BHV-1300 announced in Graves' Disease, a common autoimmune disorder affecting approximately 3 million in the US and 80 million globally. Graves' Disease is caused by IgG1 autoantibodies that hyperstimulate the TSH receptor, causing hyperthyroidism and can result in the need for surgical removal, chemical ablation of the thyroid, or need for chronic anti-thyroid drug therapy. Additional programs in rheumatoid arthritis and myasthenia gravis also to be pursued with BHV-1300 and 1310.
- Study May Proceed letter received from FDA for BHV-1310 IND, a next generation IgG degrader and Phase 1 initiating in 1H 2025. BHV-1300 and BHV-1310 are similar but will optimize therapeutic targeting and facilitate broader commercial development options.
- Next generation MoDE degrader targets advancing in 2025 include:
- IgG4 specific degrader
- PLA2R autoantibody degrader for membranous nephropathy
- Pro-insulin autoantibody degrader for type 1 diabetes
- TSH receptor autoantibody degrader as a selective follow-on asset for Graves' Disease
Upcoming milestones in the degrader program include:
- IgG MoDE Degraders (1300/1310): BHV-1300 Phase 1 completing last remaining dose cohorts with the optimized subcutaneous formulation with expected completion in 1H 2025. BHV-1310 first-in-human study anticipated to initiate 1H 2025. Phase 2 study in Graves' Disease expected to initiate mid-2025 and additional programs in rheumatoid arthritis and myasthenia gravis continue to be pursued with BHV-1300/1310.
- Phase 1 with BHV-1400 and BHV-1600 expected to be completed in 1H 2025.
- Four molecules moving towards development candidate in 2025 including: IgG4 degrader, PLA2R autoantibody degrader, insulin autoantibody degrader, and TSH receptor autoantibody degrader.
Tova Gardin, M.D., M.P.P, Biohaven Chief Translational Officer, reflected on the recent results from the MoDE degrader platform, "With the advancements across our degrader platform, including the highly selective TRAP molecules, Biohaven inaugurates a new age of immune-modulating treatment – one which opens the potential of treating the pathogenesis of disease with precision to restore healthy homeostasis. The results of BHV-1400 from the first and lowest SAD cohort highlight the speed, precision, and patient-centered innovation that drives development of each of our molecules. Lowering Gd-IgA1 by
BHV-2100: First-in-clinic, oral, selective TRPM3 antagonist that offers a novel, non-addictive treatment for migraine and neuropathic pain. Based on favorable pharmacokinetic and safety data from the Phase 1 studies in healthy subjects, a Phase 1b laser-evoked hyperalgesia trial was performed and a proof-of-concept in the acute treatment of migraine is ongoing. Preliminary data from the laser-evoked hyperalgesia study demonstrated that BHV-2100 reduced laser heat-induced pain and brain evoked potentials in healthy volunteers. This exciting result is a culmination of years of laboratory research and represents a powerful entree into the field. It provides the first indication of potential clinical efficacy in pain with the novel TRPM3 mechanism recapitulating antinociceptive preclinical efficacy across a spectrum of pain models. Data from the laser-evoked potential study and proof-of-concept migraine study expected in 1H 2025.
BHV-7000: Selective activator of Kv7.2/7.3 potassium channels, a breakthrough target in neurology and neuropsychiatry with blockbuster potential. Kv7 activation is a clinically validated target for treating mood disorders and epilepsy. Registrational studies ongoing in bipolar disorder, major depressive disorder, focal epilepsy, and generalized epilepsy.
Upcoming milestones in the BHV-7000 program include:
- Pivotal bipolar and major depressive disorder topline results expected in 1H 2025 and 2H 2025, respectively. Focal epilepsy study topline results expected in 1H 2026.
Troriluzole: Troriluzole is a novel glutamate modulator currently in Phase 3 development for Spinocerebellar ataxia (SCA) and obsessive-compulsive disorder (OCD). A new drug application (NDA) was submitted to US FDA for troriluzole in all SCA genotypes, following completion of pre-NDA meeting in 4Q 2024. Troriluzole has Orphan Drug and Fast-Track designations and qualifies for potential Priority Review. EU marketing authorization application also under review for troriluzole in all SCA genotypes. There are no FDA-approved treatments for SCA. Additionally, two Phase 3 trials with troriluzole in OCD are ongoing.
Upcoming milestones in the troriluzole program include:
- Preparing for commercial launch in SCA in 2025, while awaiting filing decision from FDA on the troriluzole all-genotype SCA NDA resubmission.
- Topline data from two Phase 3 OCD trials in 1H 2025 and 2H 2025, respectively.
Taldefgrobep alfa: Taldefgrobep is a novel myostatin inhibitor that is optimized to block both myostatin and activin A signaling, two key regulators of muscle and fat metabolism. Biohaven is studying taldefgrobep in a global Phase 3 expansion study in Spinal Muscular Atrophy (SMA), as an adjunctive therapy to enhance muscle mass and function in patients treated with standard-of-care therapies. Analyses of prespecified subgroups by race and ethnicity demonstrated that the largest study population (
Upcoming milestones in the taldefgrobep program include:
- Expected FDA meeting to discuss SMA registrational path in 1H 2025.
- Initiate taldefgrobep Phase 2 study in obesity in 1H 2025.
BHV-8000: BHV-8000 is a highly selective, oral, brain-penetrant, selective TYK2/JAK1 inhibitor with broad potential for neurodegenerative and neuroinflammatory disorders. In the Phase 1 SAD/MAD study in healthy participants, BHV-8000 was generally safe and well-tolerated while producing significant reductions in inflammatory biomarkers relative to placebo. Target indications for BHV-8000 include Parkinson's disease, Alzheimer's disease, prevention of amyloid-related imaging abnormalities (ARIA), and multiple sclerosis (MS). In 2024, Biohaven completed interactions with FDA enabling registrational programs for Parkinson's disease and the prevention of ARIA.
Upcoming milestones in the BHV-8000 program include:
- Initiate BHV-8000 Phase 2/3 study in Parkinson's disease in 1H 2025
- Advance Alzheimer's, MS and ARIA programs in 2025.
Oncology antibody drug conjugate (ADC) portfolio:
BHV-1510 (Trop2 ADC): Preliminary data from the initial Phase 1 study dosing cohorts of BHV-1510 have demonstrated promising clinical activity, including tumor shrinkage, with a tolerable safety profile of the novel topoisomerase 1 (TopoIx) payload. The Phase 1/2 study of BHV-1510 is progressing with robust enrollment in dose escalation and optimization, both as monotherapy and in combination with the anti-PD1 monoclonal antibody Libtayo® (cemiplimab-rwlc) through a clinical supply agreement with Regeneron.
- Preclinically, TopoIx has shown increased immunogenic cell death and synergistic activity when combined with anti-PD1/L1 checkpoint inhibitors, and a differentiated nonclinical toxicity profile;
- Clinical activity has been seen across dose cohorts tested to date, including the lowest dose tested of 2mg/kg Q3W;
- Early PK data demonstrates a stable ADC with very low serum concentrations of free payload;
- Preliminary safety data demonstrates a favorable profile, with no payload-associated interstitial lung disease, gastrointestinal toxicities, or significant hematological toxicities observed in early cohorts. The main toxicity observed thus far in Phase 1 study has been stomatitis, an expected on-target Trop2 class toxicity that has been manageable;
- Combination cohorts of BHV-1510 with Libtayo® have initiated.
Based on these encouraging early results, Biohaven has entered into an expanded collaboration agreement with GeneQuantum, which provides broad target exclusivity for up to 18 ADC targets incorporating the novel topoisomerase 1 inhibitor (TopoIx) payload.
Biohaven has incorporated the TopoIx payload into its next clinical-stage investigational agent, BHV-1530. BHV-1530 is an FGFR3-directed ADC with potential indications in cancers driven by FGFR3 alterations and/or upregulated FGFR3 protein expression, including urothelial cancers and other solid tumors. While FGFR3 has been clinically validated as a target in oncology, there are no other FGFR3 ADCs currently in clinical development. Biohaven retains global rights for BHV-1530 under the agreement via an exclusive license with GeneQuantum and Aimed Bio. The US IND has been opened, and a first-in-human study for solid tumors is planned for 1H 2025.
In addition, Biohaven today announced a multi-target collaboration with Merus, an oncology-focused biotechnology company developing innovative, multi-specific (Biclonics® and Triclonics®) antibodies. This collaboration will co-develop three programs encompassing highly differentiated next generation dual-targeted bispecific ADCs leveraging Biohaven's proprietary conjugation and payload technologies along with Merus' leading Biclonics technology platform.
Together, the announced milestones and collaborations represent a significant expansion of Biohaven's ADC portfolio, positioning the Company with potential to deliver highly differentiated therapeutics and address significant unmet needs in Oncology.
Nushmia Khokhar, M.D., Biohaven Chief Medical Officer of Oncology, commented, "These are exciting times for Biohaven's oncology pipeline as we are well-positioned to introduce differentiated, next generation ADCs to the clinic. The early Phase 1 data with BHV-1510 is promising, showing not only signs of clinical activity but also minimal toxicities related to the free payload. This affirms the advantages of our conjugation technology, which provides high ADC stability. The distinct profile of the novel TopoIx payload, and its potential to synergize with checkpoint inhibitor therapy, could significantly benefit patients across various cancer types. Furthermore, our collaboration with Merus and the expanded partnership with GeneQuantum—utilizing the TopoIx payload for multiple targets—demonstrate the potential of Biohaven's upcoming innovative ADCs. This includes dual-targeting ADCs, which are exciting for their potential to address challenges like tumor heterogeneity and delivery of stable ADCs with an improved therapeutic index."
Upcoming milestones in the oncology program include:
- Interim Phase 1 data with BHV-1510 and dose optimization as monotherapy and combination therapy with Libtayo® in epithelial tumors in 2025.
- Initiate Phase 1 trial of BHV-1530 in 1H 2025.
- Advance Merus collaboration ADCs (undisclosed targets) and TopoIx ADCs in 2025.
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience, and oncology. Biohaven is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's extensive clinical and nonclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable US regulatory requirements; the potential commercialization of Biohaven's product candidates; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MoDE is a trademark of Biohaven Therapeutics Ltd.
TRAP is a trademark of Biohaven Therapeutics Ltd.
Biclonics and Triclonics are registered trademarks of Merus NV.
Libtayo® is a registered trademark of Regeneron.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-portfolio-progress-innovation-and-anticipated-milestones-at-the-43rd-annual-jp-morgan-healthcare-conference-reports-positive-degrader-data-with-rapid-deep-and-selective-lowering-of-galactose-deficient-ig-302349336.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-highlights-portfolio-progress-innovation-and-anticipated-milestones-at-the-43rd-annual-jp-morgan-healthcare-conference-reports-positive-degrader-data-with-rapid-deep-and-selective-lowering-of-galactose-deficient-ig-302349336.html
SOURCE Biohaven Ltd.