Theralase(R) Demonstrates Unique Ability to Activate Rutherrin(R) With Diabetes Drug
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) has made a significant breakthrough in cancer treatment. Their lead drug formulation, Rutherrin, can now be activated by Metformin, a common diabetes drug, without the need for light or radiation. This discovery allows for precise targeting of cancer cells anywhere in the body, including the brain.
Key findings include:
- Rutherrin combined with Metformin significantly increases Reactive Oxygen Species (ROS) production in Non-Small Cell Lung Cancer (NSCLC) cells
- The combination is even more effective when activated by radiation
- This new method could enable out-patient or in-home cancer treatments
- It potentially reduces treatment costs and burden on patients with mobility
Theralase plans to commence clinical studies for various cancers, pending regulatory approval.
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) ha fatto un'importante scoperta nel trattamento del cancro. La loro formulazione di farmaco principale, Rutherrin, può ora essere attivata da Metformina, un comune farmaco per il diabete, senza la necessità di luce o radiazioni. Questa scoperta consente un targeting preciso delle cellule cancerose in qualsiasi parte del corpo, incluso il cervello.
I risultati chiave includono:
- Rutherrin combinato con Metformina aumenta significativamente la produzione di specie reattive dell'ossigeno (ROS) nelle cellule del carcinoma polmonare non a piccole cellule (NSCLC)
- La combinazione è ancora più efficace quando attivata da radiazioni
- Questo nuovo metodo potrebbe consentire trattamenti per il cancro ambulatoriali o a casa
- Potrebbe potenzialmente ridurre i costi di trattamento e il carico per i pazienti con mobilità ridotta
Theralase prevede di avviare studi clinici per vari tipi di cancro, in attesa dell'approvazione normativa.
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) ha logrado un avance significativo en el tratamiento del cáncer. Su formulación principal de fármaco, Rutherrin, ahora puede ser activada por Metformina, un medicamento común para la diabetes, sin necesidad de luz o radiación. Este descubrimiento permite un enfoque preciso en las células cancerosas en cualquier parte del cuerpo, incluido el cerebro.
Los hallazgos clave incluyen:
- Rutherrin combinado con Metformina aumenta significativamente la producción de Especies Reactivas de Oxígeno (ROS) en células de carcinoma pulmonar no microcítico (NSCLC)
- La combinación es incluso más efectiva cuando se activa mediante radiación
- Este nuevo método podría permitir tratamientos ambulatorios o en casa para el cáncer
- Potencialmente reduce los costos de tratamiento y la carga en pacientes con movilidad reducida
Theralase planea comenzar estudios clínicos para varios tipos de cáncer, a la espera de la aprobación regulatoria.
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF)는 암 치료에서 중요한 발전을 이루었습니다. 그들의 주요 약물 제형인 Rutherrin은 이제 흔한 당뇨병 약물인 메트포르민으로 빛이나 방사선 없이 활성화 될 수 있습니다. 이 발견은 뇌를 포함하여 신체의 어디에서나 암세포를 정확하게 표적할 수 있게 해줍니다.
주요 발견 사항은 다음과 같습니다:
- 메트포르민과 결합된 Rutherrin은 비소세포 폐암(NSCLC) 세포에서 반응성 산소 종(ROS) 생성을 상당히 증가시킵니다.
- 이 조합은 방사선에 의해 활성화될 때 더욱 효과적입니다.
- 이 새로운 방법은 외래환자 또는 가정에서의 암 치료를 가능하게 할 수 있습니다.
- 환자의 이동성 부담을 줄이고 치료 비용을 잠재적으로 감소시킵니다.
Theralase는 여러 암에 대한 임상 연구를 규제 승인을 기다리며 시작할 계획입니다.
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) a réalisé une avancée significative dans le traitement du cancer. Leur formulation de médicament phare, Rutherrin, peut désormais être activée par Métformine, un médicament courant pour le diabète, sans avoir besoin de lumière ou de radiation. Cette découverte permet de cibler précisément les cellules cancéreuses dans tout le corps, y compris dans le cerveau.
Les principales conclusions comprennent :
- Rutherrin combiné à la Metformine augmente significativement la production d'espèces réactives de l'oxygène (ROS) dans les cellules de cancer du poumon non à petites cellules (NSCLC)
- La combinaison est encore plus efficace lorsqu'elle est activée par la radiation
- Cette nouvelle méthode pourrait permettre des traitements contre le cancer en ambulatoire ou à domicile
- Elle pourrait réduire les coûts de traitement et le fardeau des patients ayant une mobilité réduite
Theralase prévoit de commencer des études cliniques pour divers cancers, sous réserve de l'approbation réglementaire.
Theralase Technologies Inc. (TSXV:TLT)(OTCQB:TLTFF) hat einen bedeutenden Durchbruch in der Krebsbehandlung erzielt. Ihre Hauptmedikamentenformulierung, Rutherrin, kann jetzt durch Metformin, ein übliches Diabetesmedikament, aktiviert werden, ohne dass Licht oder Strahlung benötigt wird. Diese Entdeckung ermöglicht eine präzise Zielgerichtung von Krebszellen überall im Körper, einschließlich des Gehirns.
Wichtige Erkenntnisse umfassen:
- Rutherrin in Kombination mit Metformin erhöht signifikant die Produktion reaktiver Sauerstoffspezies (ROS) in nicht-kleinzelligem Lungenkrebs (NSCLC) Zellen
- Die Kombination ist noch effektiver, wenn sie durch Strahlung aktiviert wird
- Diese neue Methode könnte ambulante oder häusliche Krebsbehandlungen ermöglichen
- Sie potenziell die Behandlungskosten und die Belastung für Patienten mit eingeschränkter Mobilität reduzieren
Theralase plant, klinische Studien für verschiedene Krebsarten zu beginnen, vorbehaltlich der behördlichen Genehmigung.
- Rutherrin can now be activated by Metformin without light or radiation
- The combination of Rutherrin and Metformin significantly increases ROS production in NSCLC cells
- The treatment can potentially target cancer cells anywhere in the body, including the brain
- The new method could enable out-patient or in-home cancer treatments
- Potential reduction in treatment costs and burden on patients with mobility
- Clinical studies for the new treatment method are still pending
- Regulatory approval is required before commencing clinical trials
TORONTO, ON / ACCESSWIRE / August 21, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation-activated small molecules for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that it's lead drug formulation, Rutherrin® has been proven preclinically to be activated by Metformin, a common diabetes drug, without the use of light and/or radiation.
Even more interesting is that because Rutherrin® and Metformin are both scientifically proven to cross the blood-brain barrier, as well as tumour-specific blood barriers, this new discovery potentially allows the precise targeting of cancer cells by Rutherrin® anywhere inside the body, including the brain, followed by their synergistic activation by Metformin.
Metformin, an anti-diabetic agent, was approved by the U.S. Food and Drug Administration in 1994 for the treatment of Type 2 diabetes (a medical condition resulting from the insufficient production of insulin, causing high blood sugar). Metformin is currently the only anti-diabetic medication prescribed for the prophylactic (intended to prevent disease) treatment of prediabetes, as recommended by the American Diabetes Association.
In order to determine if Metformin has the ability to activate Rutherrin® to produce Reactive Oxygen Species ("ROS"), for the destruction of treatment resistant, Non-Small Cell Lung Cancer ("NSCLC") cells, NSCLC cells were treated with either Rutherrin®, Metformin or a combination of both for 24 hours.
As can be seen in Figure 1.0, Rutherrin® on its own increases ROS production, without any external stimuli, leading to cancer cell death; however, this ROS production is significantly increased by activating Rutherrin® with Metformin (p<0.001).
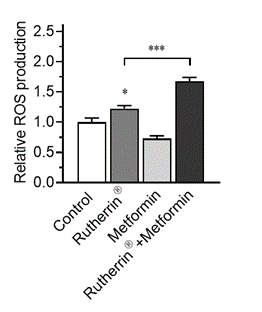
Figure 1.0: Reactive Oxygen Species Production in NSCLC Cells Treated with Rutherrin®, Metformin or Their Combination
To further explore the activation of Rutherrin® by Metformin, an additional set of experiments were conducted, where the cells were treated with either Rutherrin®, Metformin or both for 24 hours and then irradiated with radiation.
As can be seen in Figure 2.0, as expected, radiation-activated Rutherrin® increases ROS production (p<0.01); however, ROS production is significantly increased with radiation-activated Rutherrin® when combined with Metformin (p<0.001).
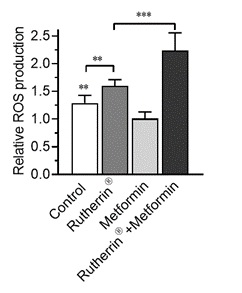
Figure 2.0: Reactive Oxygen Species Production in NSCLC Cells Treated with Radiation-Activated Rutherrin®, Metformin or Their Combination
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, "The ability to activate Rutherrin®, with commonly available drugs like Metformin, significantly opens the clinical applications of Rutherrin®, in the destruction of various cancers. Rutherrin®, when activated by light and/or radiation, leads to the production of ROS, which is one of the key Mechanisms of Action in the destruction of cancer by the compound. Synergistic activation of Rutherrin®, using a drug, such as Metformin, with or without radiation exposure, opens up a wide range of advantageous opportunities for practitioners to treat patients outside the operating room. In addition, this latest research supports a whole new line of research; specifically, identifying additional drugs, as potential synergistic candidates for activating Rutherrin® in the destruction of various cancers."
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, "The activation of Rutherrin® by Metformin is a game changer in the clinical use of Rutherrin®. By activating it, without light and/or radiation, this treatment can significantly be expanded to offering it on an out-patient basis or even an in-home treatment basis. Traditionally, Rutherrin®, is required to be activated by laser light and/or radiation, but now it is envisioned that Rutherrin® can be instilled in the body via IV drip followed by activation with Metformin, taken orally. This can be accomplished virtually anywhere, and if required, can be accompanied by hospital-based radiation treatments, if the disease state requires it. Based on the latest research, pending Good Laboratory Practices toxicology analysis of Rutherrin® and regulatory approval, Theralase® plans to commence practitioner-led and/or Theralase®-led, clinical studies in the destruction of various cancers. The clear advantage is that light and/or radiation equipment is no longer a prerequisite and the procedure can be completed outside of the hospital or expensive operating room, significantly lowering the cost of providing the treatment and the burden on elderly patients, with limited mobility. We look forward to commercializing this technology and its ease of delivery for the benefit of all patients stricken with this terrible disease."
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation and drug activated compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include, but are not limited to, statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management for future research, development and commercialization of the Company's small molecules and their drug formulations; including: preclinical research, clinical studies and development and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, whether the Company is able to: adequately fund and secure the requisite regulatory approvals to successfully complete preclinical and clinical studies in a timely fashion to implement its development plan; successfully commercialize its drug formulations; access sufficient capital to fund the Company's operations, which may not be available on terms that are commercially favorable to the Company or at all; provide preclinical and clinical support that the Company's drug formulations are effective against the conditions tested in its preclinical and clinical studies; comply with the term of license agreements with third parties, not to lose the right to use key intellectual property in its business; protect its intellectual property and the timing and success of this intellectual property and achieve acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will successfully come to fruition, and as such, FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For More Information:
1.866.THE.LASE (843.5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
khachey@theralase.com
416.699.LASE (5273) x 224
SOURCE: Theralase Technologies, Inc.
View the original press release on accesswire.com
FAQ
What is the new breakthrough announced by Theralase Technologies (TLTFF) for cancer treatment?
How does the combination of Rutherrin and Metformin affect cancer cells?
What are the potential benefits of Theralase's (TLTFF) new cancer treatment method?







