Theralase(R) Demonstrates Effective Treatment of Herpes
Theralase Technologies has validated previous University of Manitoba research, demonstrating that Ruvidar™ is safe and effective in treating Herpes Simplex Virus Type 1 (HSV-1) in an animal model. The research showed complete healing of HSV-1 cutaneous lesions in Balb/C mice after four days of treatment with a 1% Ruvidar solution.
The global HSV treatment market was valued at $2.5 billion in 2023, with an expected CAGR of 8.1% from 2024 to 2030. North America holds the largest market share at 32.4%. An estimated 3.8 billion people under age 50 globally have HSV-1, while 520 million people aged 15-49 have HSV-2.
Based on these successful results, Theralase plans to develop both a vaccine and therapeutic for HSV prevention and treatment, with clinical development to follow.
Theralase Technologies ha convalidato la precedente ricerca dell'Università del Manitoba, dimostrando che Ruvidar™ è sicuro ed efficace nel trattamento del virus Herpes Simplex di tipo 1 (HSV-1) in un modello animale. La ricerca ha mostrato una completa guarigione delle lesioni cutanee da HSV-1 nei topi Balb/C dopo quattro giorni di trattamento con una soluzione di Ruvidar all'1%.
Il mercato globale del trattamento dell'HSV è stato valutato 2,5 miliardi di dollari nel 2023, con un CAGR previsto dell'8,1% dal 2024 al 2030. L'America del Nord detiene la quota di mercato più grande con il 32,4%. Si stima che 3,8 miliardi di persone sotto i 50 anni a livello globale abbiano HSV-1, mentre 520 milioni di persone di età compresa tra 15 e 49 anni abbiano HSV-2.
Basandosi su questi risultati positivi, Theralase prevede di sviluppare sia un vaccino che un trattamento terapeutico per la prevenzione e il trattamento dell'HSV, con lo sviluppo clinico a seguire.
Theralase Technologies ha validado la investigación previa de la Universidad de Manitoba, demostrando que Ruvidar™ es seguro y efectivo en el tratamiento del virus del Herpes Simplex tipo 1 (HSV-1) en un modelo animal. La investigación mostró una curación completa de las lesiones cutáneas de HSV-1 en ratones Balb/C después de cuatro días de tratamiento con una solución de Ruvidar al 1%.
El mercado global de tratamientos para el HSV fue valorado en 2.5 mil millones de dólares en 2023, con una tasa de crecimiento anual compuesta (CAGR) esperada del 8.1% desde 2024 hasta 2030. América del Norte tiene la mayor participación de mercado con un 32.4%. Se estima que 3.8 mil millones de personas menores de 50 años a nivel mundial tienen HSV-1, mientras que 520 millones de personas de entre 15 y 49 años tienen HSV-2.
Con base en estos resultados exitosos, Theralase planea desarrollar tanto una vacuna como un tratamiento terapéutico para la prevención y el tratamiento del HSV, con el desarrollo clínico que seguirá.
Theralase Technologies는 매니토바 대학교의 이전 연구를 검증하여 Ruvidar™가 동물 모델에서 단순 포진 바이러스 1형(HSV-1) 치료에 안전하고 효과적임을 입증했습니다. 연구 결과, Balb/C 쥐에서 HSV-1 피부 병변이 1% Ruvidar 용액으로 4일 치료 후 완전히 치유되었습니다.
2023년 전 세계 HSV 치료 시장은 25억 달러로 평가되었으며, 2024년부터 2030년까지 연평균 성장률(CAGR)은 8.1%로 예상됩니다. 북미는 32.4%의 가장 큰 시장 점유율을 차지하고 있습니다. 전 세계적으로 50세 미만의 38억 명이 HSV-1에 감염되어 있으며, 5억 2천만 명이 15세에서 49세 사이에서 HSV-2를 가지고 있습니다.
이러한 성공적인 결과에 기반하여, Theralase는 HSV 예방 및 치료를 위한 백신과 치료제를 개발할 계획이며, 임상 개발이 뒤따를 것입니다.
Theralase Technologies a validé les recherches précédentes de l'Université du Manitoba, démontrant que Ruvidar™ est sûr et efficace dans le traitement du virus Herpès Simplex de type 1 (HSV-1) dans un modèle animal. La recherche a montré une guérison complète des lésions cutanées dues au HSV-1 chez des souris Balb/C après quatre jours de traitement avec une solution de Ruvidar à 1 %.
Le marché mondial du traitement de l'HSV était évalué à 2,5 milliards de dollars en 2023, avec un taux de croissance annuel composé (CAGR) prévu de 8,1 % de 2024 à 2030. L'Amérique du Nord détient la plus grande part de marché avec 32,4 %. On estime que 3,8 milliards de personnes de moins de 50 ans dans le monde ont l'HSV-1, tandis que 520 millions de personnes âgées de 15 à 49 ans ont l'HSV-2.
Sur la base de ces résultats prometteurs, Theralase prévoit de développer à la fois un vaccin et un traitement thérapeutique pour la prévention et le traitement de l'HSV, avec un développement clinique à suivre.
Theralase Technologies hat die vorherige Forschung der Universität Manitoba validiert und nachgewiesen, dass Ruvidar™ in einem Tiermodell sicher und effektiv zur Behandlung des Herpes-Simplex-Virus Typ 1 (HSV-1) ist. Die Forschung zeigte eine vollständige Heilung der HSV-1-Hautläsionen bei Balb/C-Mäusen nach vier Tagen Behandlung mit einer 1%igen Ruvidar-Lösung.
Der globale Markt für HSV-Behandlungen wurde im Jahr 2023 auf 2,5 Milliarden Dollar geschätzt, mit einer erwarteten jährlichen Wachstumsrate (CAGR) von 8,1% von 2024 bis 2030. Nordamerika hält den größten Marktanteil mit 32,4%. Schätzungsweise 3,8 Milliarden Menschen unter 50 Jahren weltweit haben HSV-1, während 520 Millionen Menschen im Alter von 15 bis 49 Jahren HSV-2 haben.
Basierend auf diesen erfolgreichen Ergebnissen plant Theralase die Entwicklung sowohl eines Impfstoffs als auch einer Therapie zur Prävention und Behandlung von HSV, gefolgt von der klinischen Entwicklung.
- Complete healing of HSV-1 lesions achieved in animal model after 4 days of treatment
- Company entering large market valued at $2.5B with 8.1% CAGR (2024-2030)
- Potential to address massive market of 3.8B people affected by HSV-1
- Still in early research phase with only animal model results
- Clinical development yet to begin
- Will face competition in established market with existing treatments
Theralase® validates previous University of Manitoba research by demonstrating that RuvidarTM is safe and effective in the treatment of the Herpes Simplex Virus in an animal model.
TORONTO, ON / ACCESS Newswire / February 12, 2025 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that the previous University of Manitoba research has now been validated, proving that RuvidarTM is safe and effective in the inactivation of Herpes Simplex Virus, Type 1 ("HSV-1") in an animal model.
Herpes Simplex Virus ("HSV"), known as herpes, is a common infection that can cause painful blisters or ulcers. It primarily spreads by skin-to-skin contact. It is treatable but not curable.1
There are two types of HSV: 1
Type 1 ("HSV-1") mostly spreads by oral contact and causes infections in or around the mouth (oral herpes or cold sores). It can also cause genital herpes. Most adults are infected with HSV-1.
Type 2 ("HSV-2") spreads by sexual contact and causes genital herpes.
An estimated 3.8 billion people under age 50 (
The global HSV treatment market size was estimated at $USD 2.5 billion in 2023 and is expected to grow at a Compound Annual Growth Rate ("CAGR") of
The market growth can be attributed to the growing concerns over HSV infection, including, oral and genital herpes. Moreover, the infection is highly contagious, spreading via saliva, vaginal secretion or semen and is acquired unknowingly. These factors highlight the increasing need for treatment throughout the projected period.2
North America accounted for the largest market share of
The HSV-1 lifecycle begins upon contact with mucosal surfaces and it is in this niche, where the virus actively replicates inducing local lesion formation. The virus then enters local sensory nerve endings and migrates back to neuronal cell bodies in the peripheral nervous system. It is in this location where the virus enters into a latent, non-replicative stage until later reactivation.3
The ability of HSV-1 to infect and establish latency in neurons allows for lifelong infection and can provide the virus with access to other sites such as the central nervous system. Recent research has implicated HSV-1 infection with the development of disease later in life, including Parkinson's and Alzheimer's diseases.4,5
Similarly, reactivation of HSV-1 in autonomic nerves that innervate coronary arteries may introduce lytic virus to vascular endothelial cells, causing local injury, thrombosis and arteriosclerosis, as well as potentially contributing to various other cardiovascular disorders.6,7
Evidence has also accumulated indicating that, in addition, HSV may be a cause of human cancers.8
Despite longstanding attempts at therapy and prevention, HSV remains among the most prevalent human infectious viral pathogens; therefore, it's imperative to keep HSV from replicating by implementing advanced vaccines and more effective drugs to combat and defeat this pervasive scourge to the human race.
In the latest Theralase® research, Balb/C mice were infected with human HSV-1 virus.
On day 6 post-infection, 20 uL of
Four days of Ruvidar® treatment resulted in complete healing of the HSV-1 cutaneous lesions.
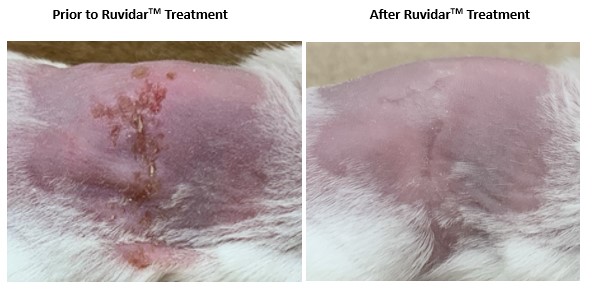
Figure 1. Four days of Ruvidar® treatment in Balb/C mice with HSV-1 infected cutaneous lesions
The results support the safety and efficacy of topically applied non-light activated Ruvidar® against cutaneous HSV-1 lesions in a mouse model.
Kevin Coombs, B.A., M.A., Ph.D., professor of medical microbiology and infectious diseases at the Max Rady College of Medicine, University of Manitoba (retired) stated, "I am delighted that Theralase® researchers were able to successfully translate my team's cellular inactivation of HSV into a safe and effective therapy in an animal model. Their research may prove to be instrumental in the development of a clinical program that will have real world impacts on the lives of billions of people infected with this prolific disease."
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer, Theralase® stated, "I am excited by the ground-breaking results of our first animal experiments in which we successfully treated laboratory mice with HSV-1. Herpes is an extremely difficult disease to treat, as it prefers to reside inactive in nerve cells and then reawaken due to various stimuli (i.e., illness, fever, sun exposure, menstrual period, injury, emotional stress or surgery) to induce painful and unsightly skin lesions. It has also been linked with the development of chronic disease, such as cardiovascular disorders and cancer. Many people living with HSV are concerned about the risk of transmitting the disease to other people. Our goal, as we advance into clinical development, is to safely and effectively treat people with HSV infections, so that they are less self-conscious of outbreaks and the risk of transmitting the virus to another person. I hope this research lays the groundwork for Theralase® to provide therapy to safely and effectively treat herpes."
Roger DuMoulin-White, B.Sc., P.Eng, Pro.Dir., President and Chief Executive Officer, Theralase® stated, "Based on the success of Theralase®'s latest research, Theralase® plans to develop a vaccine and therapeutic for the prevention and treatment of HSV, with clinical development to commence thereafter."
References:
2 Herpes Simplex Virus Treatment Market Size, Share & Trends Analysis Report By Type (HSV-1, HSV-2), By Drug (Acyclovir, Valacyclovir, Famciclovir), By Vaccine (Simplirix, Others), By Route of Administration, By End-use, By Region, And Segment Forecasts, 2024 - 2030
Roizman B, Knipe DM, Whitley R. Herpes Simplex Viruses. 6th ed. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1823-1897.
Mangold CA, Szpara ML. Persistent infection with herpes simplex virus 1 and Alzheimer's disease-a call to study how variability in both virus and host may impact disease. Viruses. 2019;11: 966. pmid:31635156.
Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci. 1983;80: 6386-6389. pmid:6312457.
Phelps A, Gates AJ, Eastaugh L, Hillier M, Ulaeto DO. Comparative Efficacy of Intramuscular and Scarification Routes of Administration of Live Smallpox Vaccine in a Murine Challenge Model. Vaccine. 2017 Jul 5;35(31):3889-3896. doi: 10.1016/j.vaccine.2017.05.058. Epub 2017 Jun 9.
Shchelkunov SN, Sergeev AA, Pyankov SA, Titova KA, Yakubitskiy SN. Smallpox vaccination in a mouse model. Vavilovskii Zhurnal Genet Selektsii. 2023 Oct;27(6):712-718. doi: 10.18699/VJGB-23-82.
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all; the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies; the risk that the Company fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com
SOURCE: Theralase Technologies, Inc.
View the original press release on ACCESS Newswire







