Ruvidar Effective in the Treatment of Herpes
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF) has announced promising results for their drug Ruvidar™ in treating Herpes Simplex Virus Type 1 (HSV-1). In preclinical animal trials, Ruvidar demonstrated superior efficacy compared to standard treatments Acyclovir and Abreva.
The global HSV treatment market, valued at $2.8 billion in 2024, is projected to reach $4.7 billion by 2033, with North America holding a 37.1% market share. An estimated 3.8 billion people globally have HSV-1, highlighting significant market potential.
The research showed Ruvidar's unique mechanism of action, utilizing positive charge to block viral glycoproteins and prevent virus replication. Following these successful preclinical results, Theralase plans to begin formulating Ruvidar into topical form and commence Phase I/II adaptive clinical studies in 2025, pending funding, to evaluate its safety and efficacy in treating cold sore lesions in humans.
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF) ha annunciato risultati promettenti per il loro farmaco Ruvidar™ nel trattamento del virus Herpes Simplex di tipo 1 (HSV-1). Negli studi preclinici su animali, Ruvidar ha dimostrato un'efficacia superiore rispetto ai trattamenti standard Acyclovir e Abreva.
Il mercato globale del trattamento dell'HSV, valutato 2,8 miliardi di dollari nel 2024, è previsto raggiungere 4,7 miliardi di dollari entro il 2033, con il Nord America che detiene una quota di mercato del 37,1%. Si stima che 3,8 miliardi di persone nel mondo siano affette da HSV-1, evidenziando un significativo potenziale di mercato.
La ricerca ha mostrato il meccanismo d'azione unico di Ruvidar, che utilizza una carica positiva per bloccare le glicoproteine virali e prevenire la replicazione del virus. Dopo questi risultati preclinici di successo, Theralase prevede di iniziare a formulare Ruvidar in forma topica e di avviare studi clinici adattivi di Fase I/II nel 2025, in attesa di finanziamenti, per valutare la sua sicurezza e efficacia nel trattamento delle lesioni da herpes labiale negli esseri umani.
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF) ha anunciado resultados prometedores para su medicamento Ruvidar™ en el tratamiento del virus del herpes simple tipo 1 (HSV-1). En ensayos preclínicos en animales, Ruvidar demostró una eficacia superior en comparación con los tratamientos estándar Acyclovir y Abreva.
El mercado global de tratamientos para el HSV, valorado en 2.8 mil millones de dólares en 2024, se proyecta que alcanzará 4.7 mil millones de dólares para 2033, con América del Norte manteniendo una cuota de mercado del 37.1%. Se estima que 3.8 mil millones de personas en todo el mundo tienen HSV-1, lo que resalta un potencial de mercado significativo.
La investigación mostró el mecanismo de acción único de Ruvidar, que utiliza carga positiva para bloquear las glicoproteínas virales y prevenir la replicación del virus. Tras estos resultados preclínicos exitosos, Theralase planea comenzar a formular Ruvidar en forma tópica y comenzar estudios clínicos adaptativos de Fase I/II en 2025, a la espera de financiamiento, para evaluar su seguridad y eficacia en el tratamiento de lesiones por herpes labial en humanos.
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF)는 단순 포진 바이러스 타입 1 (HSV-1) 치료를 위한 약물 Ruvidar™의 유망한 결과를 발표했습니다. 동물의 전임상 시험에서 Ruvidar는 표준 치료제인 Acyclovir 및 Abreva에 비해 우수한 효능을 보여주었습니다.
2024년 28억 달러로 평가되는 전 세계 HSV 치료 시장은 2033년까지 47억 달러에 이를 것으로 예상되며, 북미는 37.1%의 시장 점유율을 차지하고 있습니다. 전 세계적으로 약 38억 명이 HSV-1에 감염되어 있어 상당한 시장 잠재력을 강조합니다.
연구 결과 Ruvidar의 독특한 작용 메커니즘이 밝혀졌으며, 이는 양전하를 이용해 바이러스의 당단백질을 차단하고 바이러스 복제를 방지합니다. 이러한 성공적인 전임상 결과에 따라 Theralase는 Ruvidar를 국소 형태로 제조하고 2025년에는 자금을 기다리며 사람의 구순포진 병변 치료를 위한 안전성과 효능을 평가하기 위해 1/2상 적응형 임상 연구를 시작할 계획입니다.
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF) a annoncé des résultats prometteurs pour son médicament Ruvidar™ dans le traitement du virus Herpès Simplex de type 1 (HSV-1). Lors des essais précliniques sur des animaux, Ruvidar a démontré une efficacité supérieure par rapport aux traitements standards Acyclovir et Abreva.
Le marché mondial des traitements de l'HSV, évalué à 2,8 milliards de dollars en 2024, devrait atteindre 4,7 milliards de dollars d'ici 2033, avec l'Amérique du Nord détenant une part de marché de 37,1%. Environ 3,8 milliards de personnes dans le monde sont atteintes de HSV-1, ce qui souligne un potentiel de marché significatif.
La recherche a montré le mécanisme d'action unique de Ruvidar, utilisant une charge positive pour bloquer les glycoprotéines virales et empêcher la réplication du virus. Suite à ces résultats précliniques réussis, Theralase prévoit de commencer à formuler Ruvidar sous forme topique et de lancer des études cliniques adaptatives de Phase I/II en 2025, sous réserve de financement, pour évaluer sa sécurité et son efficacité dans le traitement des lésions de boutons de fièvre chez l'homme.
Theralase Technologies (TSXV: TLT) (OTCQB: TLTFF) hat vielversprechende Ergebnisse für ihr Medikament Ruvidar™ zur Behandlung des Herpes-simplex-Virus Typ 1 (HSV-1) bekannt gegeben. In präklinischen Tierversuchen zeigte Ruvidar eine überlegene Wirksamkeit im Vergleich zu den Standardbehandlungen Acyclovir und Abreva.
Der globale Markt für HSV-Behandlungen, der 2024 auf 2,8 Milliarden Dollar geschätzt wird, wird bis 2033 voraussichtlich 4,7 Milliarden Dollar erreichen, wobei Nordamerika einen Marktanteil von 37,1% hält. Schätzungsweise 3,8 Milliarden Menschen weltweit sind von HSV-1 betroffen, was das erhebliche Marktpotential unterstreicht.
Die Forschung zeigte den einzigartigen Wirkmechanismus von Ruvidar, der eine positive Ladung nutzt, um virale Glykoproteine zu blockieren und die Virusreplikation zu verhindern. Nach diesen erfolgreichen präklinischen Ergebnissen plant Theralase, Ruvidar in topischer Form zu formulieren und 2025 adaptive klinische Studien der Phase I/II zu beginnen, vorbehaltlich der Finanzierung, um dessen Sicherheit und Wirksamkeit bei der Behandlung von Lippenherpesläsionen beim Menschen zu bewerten.
- Ruvidar demonstrated superior efficacy vs standard treatments (Acyclovir and Abreva) in preclinical trials
- Unique mechanism of action showing ability to block viral replication
- Targeting large market opportunity ($2.8B in 2024, expected to reach $4.7B by 2033)
- Advancing to human clinical trials with clear development pathway
- Clinical development progress dependent on securing funding in 2025
- Still in early preclinical stage, requiring extensive testing before commercialization
- Faces competition from established treatments in a crowded market
Ruvidar(TM) demonstrates higher efficacy in the treatment of Herpes Simplex Virus, Type 1 versus the standard of care treatments Acyclovir and Abreva in a preclinical animal model.
Toronto, Ontario--(Newsfile Corp. - March 24, 2025) - Theralase® Technologies Inc. (TSXV: TLT) (OTCQB: TLTFF) ("Theralase®" or the "Company"), a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that RuvidarTM has demonstrated a higher efficacy in the treatment of Herpes Simplex Virus, Type 1 ("HSV-1") versus standard of care treatments Acyclovir (
Herpes Simplex Virus ("HSV"), known as herpes, is a very common infection that can cause painful blisters or ulcers on the skin of an individual. It primarily spreads by skin-to-skin contact, while it is treatable, it is not curable.1
There are two main types of HSV:1
Type 1 ("HSV-1") generally spreads by oral contact and causes infections in or around the mouth, vermilion, upper or lower lip region (oral herpes or cold sores). It can also cause genital herpes. A majority of adults are infected with HSV-1.
Type 2 ("HSV-2") spreads by sexual contact and causes herpes in the genital region of an individual.
An estimated 3.8 billion people under the age of 50 (
The global HSV treatment market size was estimated at $USD 2.8 billion in 2024 and is expected to balloon to $USD 4.7 billion by 2033.2
The market growth can be attributed to the growing concerns over HSV infection, including, oral and genital herpes. Moreover, the infection is highly contagious, spreading via saliva, vaginal secretion or semen and is acquired unknowingly. These factors highlight the increasing need for treatment throughout the projected period.3
North America accounted for the largest market share of
The HSV-1 lifecycle begins upon contact with mucosal surfaces and it is in this niche, where the virus actively replicates inducing local lesion formation. The virus then enters local sensory nerve endings and migrates back to neuronal cell bodies in the peripheral nervous system. It is in this location where the virus enters into a latent, non-replicative stage until later reactivation.4
Despite longstanding attempts at therapy and prevention, HSV remains among the most prevalent human infectious viral pathogens; therefore, it's imperative to keep HSV from replicating by implementing advanced vaccines and more effective drugs to combat and defeat this pervasive disease.
In the latest Theralase® research, Balb/C mice were infected with human HSV-1 virus on Day 0.
On Day 1 post-infection, these mice were treated once daily for 5 days with either: No Treatment, Abreva (a common over the counter treatment), Acyclovir (
No Treatment

Figure 1. No treatment of HSV-1 infected cutaneous lesions
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/245623_9033d9eec4e25b80_001full.jpg
Abreva Treatment

Figure 2. Abreva treatment of HSV-1 infected cutaneous lesions
To view an enhanced version of this graphic, please visit:
https://images.newsfilecorp.com/files/2786/245623_9033d9eec4e25b80_003full.jpg
Acyclovir (

Figure 3. Acyclovir (
Cannot view this image? Visit:
https://images.newsfilecorp.com/files/2786/245623_3.jpg
RuvidarTM (
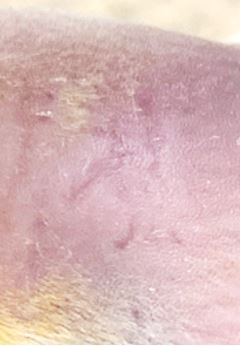
Figure 4. RuvidarTM (
Cannot view this image? Visit:
https://images.newsfilecorp.com/files/2786/245623_4.jpg
The results support the safety and efficacy of topically applied non-light activated Ruvidar® for accelerated healing of cutaneous HSV-1 lesions in a mouse model.
Pavel Kaspler, Ph.D., research scientist, Theralase®, who conducted the preclinical study stated, "I am extremely impressed with the efficacy of the RuvidarTM to successfully heal the HSV-1 lesions in an animal model versus common standard of care treatments, currently available, such as Abreva and Acyclovir. My next set of experiments will be to increase the number of daily applications of Abreva, Acyclovir and RuvidarTM (from once daily to 5 times daily) and increase the dosage of Acyclovir and RuvidarTM (1 to
Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer, Theralase® stated, "Based on the chemical properties of RuvidarTM, I am not surprised that it has had such a dramatic effect on the inactivation of HSV-1 lesions in this animal model. It is well established in the literature that the HSV-1 virus' glycoproteins (glycans - gB and gC) are negatively charged, as is the Heparan Sulphate ("HS") receptors on a cell's surface (preferred binding site of the virus on a cell). This provides a novel mechanism (based on controlled electrostatic repulsion) that addresses how viruses balance between optimized viral attachment to target cells and efficient egress of progeny virus.5,6 On the other hand, RuvidarTM is positively charged.7 This allows RuvidarTM the unique ability to be able to bind to and block the glycoproteins on HSV-1, preventing binding to host cells, as well as on the HS cell surface receptors preventing the efficient egress of progeny virus. This leads to an inability of the virus to replicate, allowing accelerated healing of cold sore lesions."
Roger DuMoulin-White, B.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer, Theralase® stated, "As always, I am in awe of the ability of RuvidarTM to effectively destroy cancer cells, as well as efficiently inactivate bacteria and viruses. Based on the success of this latest preclinical research, Theralase®, pending funding in 2025, will commence formulation of RuvidarTM into topical form, complete GLP toxicology and commence a Phase I/II adaptive clinical study to demonstrate the safety and efficacy of RuvidarTM in the accelerated healing of cold sore lesions in humans."
References:
1 Herpes simplex virus
2 Herpes Simplex Virus Treatment Market Size, Top Share, Key Developments | By 2033
3 Herpes Simplex Virus Treatment Market Size, Share & Trends Analysis Report By Type (HSV-1, HSV-2), By Drug (Acyclovir, Valacyclovir, Famciclovir), By Vaccine (Simplirix, Others), By Route of Administration, By End-use, By Region, And Segment Forecasts, 2024 - 2030
4 Roizman B, Knipe DM, Whitley R. Herpes Simplex Viruses. 6th ed. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 1823-1897.
5 Transforms of Cell Surface Glycoproteins Charge Influences Tumor Cell Metastasis via Atypically Inhibiting Epithelial-Mesenchymal Transition Including Matrix Metalloproteinases and Cell Junctions. Mingzhe Wang et al. Bioconjugate Chemistry. Vol. 34. Issue 8. July 2023
6 Olofsson S, Bally M, Trybala E, Bergström T. Structure and Role of O-Linked Glycans in Viral Envelope Proteins. Annu Rev Virol. 2023 Sep 29;10(1):283-304. doi: 10.1146/annurev-virology-111821-121007. Epub 2023 Jul 6. PMID: 37285578.
7 Ruvidar(TM) Enhances Efficacy of Cancer Drug
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light, radiation, sound and/or drug-activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedarplus.ca.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements:
This news release contains Forward-Looking Statements ("FLS") within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. FLS may be identified by the use of the words "may, "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of the Company's management regarding future research, development and commercialization of the Company's small molecules; their drug formulations; preclinical research; clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations; the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all; the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies; the risk that the Company fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business; the Company's ability to protect its intellectual property; the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these FLS, which are not a guarantee of future performance. There can be no assurance that FLS will prove to be accurate as such FLS involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the FLS.
Although the FLS contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these FLS.
All FLS are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such FLS.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer X 224
khachey@theralase.com

To view the source version of this press release, please visit https://www.newsfilecorp.com/release/245623







