Tharimmune Presents Favorable TH104 Phase 1 Clinical Data at the American College of Gastroenterology 2024 Annual Scientific Meeting
Tharimmune (NASDAQ:THAR) presented favorable Phase 1 clinical data for TH104, its lead candidate for moderate-to-severe pruritus in chronic liver disease, at the American College of Gastroenterology 2024 Annual Scientific Meeting. The single-dose trial showed no opioid withdrawal effects and demonstrated a mean 33.3% reduction in itch scores 24 hours post-dose across all subjects. The study, conducted in two cohorts of patients with different liver disease severities, reported no serious adverse events. The company plans to initiate a Phase 2 multiple-ascending dose trial with topline data expected in 2025.
Tharimmune (NASDAQ:THAR) ha presentato dati clinici favorevoli di Fase 1 per TH104, il suo principale candidato per il prurito da moderato a grave nella malattia epatica cronica, durante il Congresso Scientifico Annuale del American College of Gastroenterology 2024. Lo studio con dose singola non ha mostrato effetti di astinenza da oppioidi e ha dimostrato una riduzione media del 33,3% dei punteggi del prurito 24 ore dopo la somministrazione in tutti i soggetti coinvolti. Lo studio, condotto su due gruppi di pazienti con diverse gravità della malattia epatica, non ha riportato eventi avversi gravi. L'azienda prevede di avviare una fase 2 di uno studio con dosi multiple in aumento, con i dati principali attesi nel 2025.
Tharimmune (NASDAQ:THAR) presentó datos clínicos favorables de Fase 1 para TH104, su principal candidato para el prurito moderado a severo en la enfermedad hepática crónica, en la Reunión Científica Anual del American College of Gastroenterology 2024. El ensayo de dosis única no mostró efectos de abstinencia de opioides y demostró una reducción media del 33,3% en las puntuaciones de picazón 24 horas después de la dosis en todos los sujetos. El estudio, realizado en dos cohortes de pacientes con diferentes severidades de enfermedad hepática, no reportó eventos adversos graves. La empresa planea iniciar un ensayo de fase 2 con dosis múltiples ascendentes, con datos preliminares esperados para 2025.
Tharimmune (NASDAQ:THAR)는 TH104의 1상 임상 데이터가 만성 간 질환에서 중등도에서 중증 가려움증을 위한 주 후보물질로서 2024년 미국 소화기학회 연례 과학 회의에서 긍정적으로 발표되었습니다. 단일 용량 시험에서는 오피오이드 금단 효과가 없었으며, 모든 피험자에서 투여 24시간 후 가려움증 점수가 평균 33.3% 감소한 것으로 나타났습니다. 서로 다른 간 질환 심각도를 가진 두 개의 환자 집단에서 진행된 연구에서는 심각한 부작용이 보고되지 않았습니다. 이 회사는 2025년 예상되는 주요 데이터를 포함한 다중 상승 용량의 2상 시험을 시작할 계획입니다.
Tharimmune (NASDAQ:THAR) a présenté des données cliniques favorables de Phase 1 pour TH104, son principal candidat pour le prurit modéré à sévère dans les maladies hépatiques chroniques, lors de la Réunion Scientifique Annuelle du American College of Gastroenterology 2024. L'essai à dose unique n'a montré aucun effet de sevrage aux opioïdes et a démontré une réduction moyenne de 33,3 % des scores de démangeaisons 24 heures après la dose chez tous les sujets. L'étude, menée sur deux cohortes de patients présentant des gravités de maladies hépatiques différentes, n'a rapporté aucun événement indésirable grave. La société prévoit de lancer une étude de phase 2 avec des doses multiples croissantes, avec des données préliminaires attendues en 2025.
Tharimmune (NASDAQ:THAR) hat günstige klinische Daten der Phase 1 für TH104, seinen Hauptkandidaten bei mäßigem bis schwerem Juckreiz bei chronischer Lebererkrankung, auf dem Jahreswissenschaftlichen Treffen des American College of Gastroenterology 2024 vorgestellt. Die Einzeldosisstudie zeigte keine Entzugseffekte von Opioiden und wies eine durchschnittliche Reduktion der Juckreizbewertungen von 33,3 % 24 Stunden nach der Dosis bei allen Probanden auf. Die Studie, die in zwei Kohorten von Patienten mit unterschiedlichem Schweregrad der Lebererkrankung durchgeführt wurde, meldete keine schweren unerwünschten Ereignisse. Das Unternehmen plant, eine Phase-2-Studie mit mehrfach steigenden Dosen zu starten, wobei die Gesamtdaten im Jahr 2025 erwartet werden.
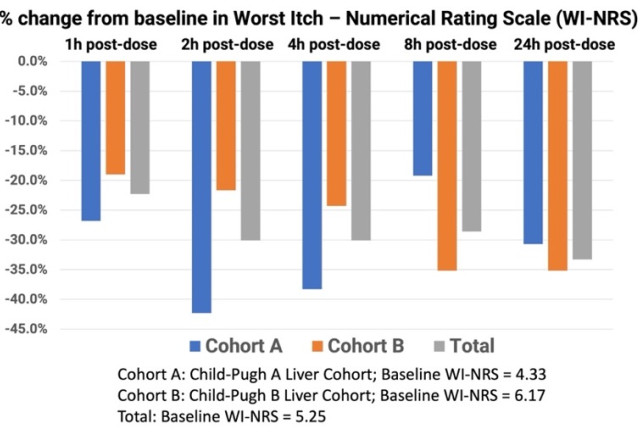
- TH104 demonstrated 33.3% mean reduction in itch scores after 24 hours from a single dose
- No serious adverse events or safety concerns reported in Phase 1 trial
- Phase 2 trial advancement planned with topline data expected in 2025
- Successful demonstration of drug delivery system effectiveness
- None.
Insights
The Phase 1 clinical trial data for TH104 shows promising results for treating pruritus in chronic liver disease patients. Key findings include: 33.3% mean reduction in itch scores across all subjects 24 hours post-dose, with improvements seen as early as 1-2 hours after administration. The drug demonstrated a favorable safety profile with no serious adverse events and notably, no opioid withdrawal effects.
The trial's design targeting both Child-Pugh A and B patients is strategically sound, covering mild to moderate liver disease cases. The proprietary microparticle embedded transmucosal delivery system shows effective drug delivery, while the rapid onset of action could differentiate TH104 from existing treatments. The progression to Phase 2 trials, with topline data expected in 2025, represents a significant milestone in addressing an unmet medical need in PBC patients.
This clinical data strengthens Tharimmune's market position in the inflammation and immunology space. The rapid symptom relief and unique delivery system could provide a competitive advantage in the pruritus treatment market. The focus on PBC patients represents a strategic entry into a specialized market segment with treatment options.
The company's engagement with both U.S. and EU regulatory authorities suggests a comprehensive commercialization strategy. The positive safety profile and efficacy data could accelerate the regulatory pathway, potentially leading to faster market entry. For a company with a market cap of
TH104, the Company's lead candidate for moderate-to-severe pruritus in chronic liver disease, showed no opioid withdrawal effects, reinforcing safety profile
TH104 was well tolerated with no unexpected treatment-emergent adverse events, supporting further clinical development
Company expects Phase 2 topline data for chronic pruritus in primary biliary cholangitis (PBC) patients in 2025
BRIDGEWATER, NJ / ACCESSWIRE / October 28, 2024 / Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company developing a portfolio of therapeutic candidates in inflammation and immunology, presented new TH104 Phase 1 data at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting, underway in Philadelphia. The Phase 1 trial was a single-dose, single-center, open-label, randomized study of TH104 transmucosal buccal film conducted in two cohorts of patients with chronic liver disease (CLD). The primary outcome measure was to determine the safety and tolerability of a single buccal dose of TH104 in these patients.
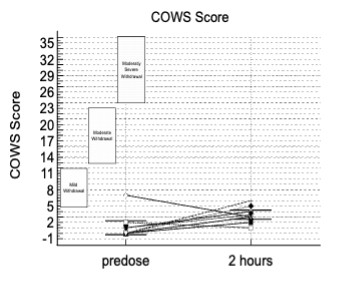
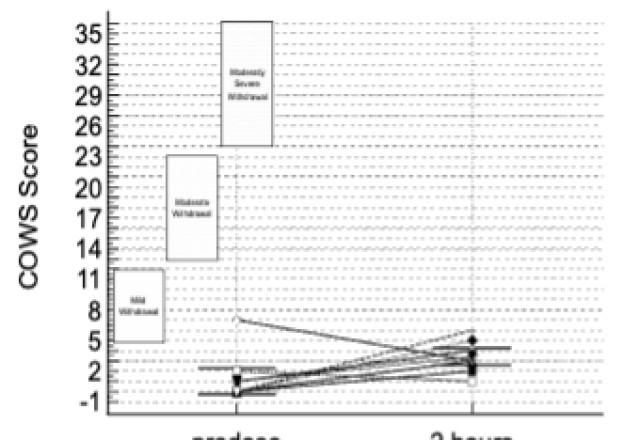
The data presented included adverse events (AEs) as well as assessment of patients using the Clinical Opiate Withdrawal Scale (COWS). The COWS is an 11-item clinician-administered scale that can be used in both inpatient and outpatient settings to reproducibly rate common signs and symptoms of opiate withdrawal and monitor them over time. The summed score for the complete scale can be used by clinicians to determine the stage or severity of withdrawal, and to assess the level of any physical dependence.
This study enrolled two types of CLD patients categorized as Child-Pugh A (cohort A) and Child-Pugh B (cohort B). The Child-Pugh score is a system for assessing the prognosis and necessity of transplant in CLD that provides a forecast of the increasing severity of a patient's liver disease and expected survival rate. The score is determined by scoring clinical measures of liver disease and the possibility of eventual liver failure, with Class A indicating mild liver disease and Class B indicating moderate liver disease with a one-to-five-year survival rates of
The mean baseline WI-NRS scores in Groups A and B were 4.33 and 6.17, respectively, translating to moderate-to-severe chronic pruritus at the start of the study. The mean baseline itch score for all 12 subjects was 5.25. At one hour post-dosing with TH104, Group A and Group B had a mean decline in WI-NRS scores of
There were no deaths, other serious adverse events or other significant adverse events reported during the entire study. There were no new adverse events during the entire study, with events correlated with previous studies and a safety profile consistent with the literature for the active ingredient in TH104.
"We are pleased with the totality of the Phase 1 data with TH104, which build upon previous studies demonstrating reliable and predictable delivery of nalmefene using our proprietary microparticle embedded transmucosal delivery system. This system is easily applied to the inside of the cheek within seconds in healthy volunteers. The mean
The Company plans to initiate a Phase 2 multiple-ascending dose trial in the coming months to assess the safety and tolerability of TH104, which will assess the change from baseline in WI-NRS scores to evaluate chronic pruritus in PBC patients. The Company expects topline data in 2025 and is engaging with both U.S. and EU regulatory authorities.
About TH104
TH104 is embedded with nalmefene onto a proprietary transdermal buccal film that easily adheres to the inside of the mouth. This endows TH104 with key features making it an ideal product candidate for multiple liver-related and other pruritogenic inflammatory conditions. The molecule has a dual mechanism of action affecting both the µ-opioid receptor and the kappa-opioid receptor, as well as potentially inhibiting IL-17 inflammatory cytokine expression. These opioid receptors when stimulated and/or inhibited by the body's natural ligands have been known to be involved in the body's itch circuitry.
About Pruritus and Primary Biliary Cholangitis
According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health, PBC is a chronic disease where the bile ducts in the liver eventually become dysfunctional and cause the buildup of bile, resulting in liver damage. The disease, believed to be an autoimmune condition, affects an estimated 58 out of every 100,000 U.S. women and about 15 out of every 100,000 U.S. men. Pruritus is one of the most common conditions associated with PBC, affecting up to
About Tharimmune
Tharimmune, Inc. is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology and inflammation. The lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor, offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multi-specific biologics targeting unique epitopes against multiple solid tumors. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding Tharimmune's or Intract's future financial or operating performance, the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's and Intract's expectations with respect to the Merger, including the timing of entering into a definitive agreement, the timing of closing thereof, the pro forma ownership of the combined company, anticipated financing plans, the combined company's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such forward-looking statements are based on the beliefs of management, as well as assumptions made by, and information currently available to, Tharimmune and Intract's management. Tharimmune may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by Tharimmune from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent Tharimmune's and Intract's views as of the date of this release. Subsequent events and developments may cause Tharimmune's views to change; however, Tharimmune does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing Tharimmune's views as of any date subsequent to the date of this release.
Contacts:
Tharimmune, Inc.
ir@tharimmune.com
Alliance Advisors IR
Tirth T. Patel
tpatel@allianceadvisors.com
212-201-6614
Contact Information
Tirth Patel
LHA Investor Relations
tpatel@lhai.com
1-212-201-6614
Related Images
|
SOURCE: Tharimmune, Inc.
View the original press release on accesswire.com