Tharimmune Announces Positive Target Engagement with Novel Biparatopic PD-1/VEGF and Multispecific HER2/HER3 Biologics Leveraging Proprietary EpiClick Technology
Tharimmune (NASDAQ:THAR) has expanded its product pipeline with HS1940, a novel biparatopic biologic targeting both PD-1 and VEGF receptors using their proprietary EpiClick Technology. HS1940 is designed to simultaneously engage the PD-1 pathway and inhibit angiogenesis, potentially offering improved treatment options for multiple cancer types.
The company has generated positive target engagement data showing that the biparatopic format provides enhanced therapeutic versatility compared to monospecific antibodies. Tharimmune plans to initiate IND-enabling studies for HS1940 throughout 2025.
Additionally, Tharimmune is developing HS3215, a multispecific antibody targeting HER2 and HER3 cancer drivers. This approach leverages unique epitopes not addressed by existing drugs, potentially overcoming resistance mechanisms associated with current HER2-targeted therapies.
Tharimmune (NASDAQ:THAR) ha ampliato il suo portafoglio prodotti con HS1940, un biologico biparatopico innovativo che mira sia ai recettori PD-1 che VEGF utilizzando la loro tecnologia proprietaria EpiClick Technology. HS1940 è progettato per attivare simultaneamente il percorso PD-1 e inibire l'angiogenesi, offrendo potenzialmente opzioni di trattamento migliorate per diversi tipi di cancro.
L'azienda ha generato dati positivi sull'impegno del target, dimostrando che il formato biparatopico offre una versatilità terapeutica superiore rispetto agli anticorpi monospecifici. Tharimmune prevede di avviare studi abilitanti IND per HS1940 nel corso del 2025.
Inoltre, Tharimmune sta sviluppando HS3215, un anticorpo multispecifico che mira ai fattori di cancro HER2 e HER3. Questo approccio sfrutta epitopi unici non affrontati dai farmaci esistenti, potenzialmente superando i meccanismi di resistenza associati alle attuali terapie mirate a HER2.
Tharimmune (NASDAQ:THAR) ha ampliado su cartera de productos con HS1940, un biológico biparatópico novedoso que se dirige tanto a los receptores de PD-1 como a los de VEGF utilizando su tecnología patentada EpiClick Technology. HS1940 está diseñado para involucrar simultáneamente la vía de PD-1 e inhibir la angiogénesis, lo que podría ofrecer opciones de tratamiento mejoradas para múltiples tipos de cáncer.
La compañía ha generado datos positivos sobre el compromiso del objetivo, mostrando que el formato biparatópico proporciona una versatilidad terapéutica mejorada en comparación con los anticuerpos monospecíficos. Tharimmune planea iniciar estudios habilitantes de IND para HS1940 a lo largo de 2025.
Además, Tharimmune está desarrollando HS3215, un anticuerpo multispecífico que se dirige a los impulsores del cáncer HER2 y HER3. Este enfoque aprovecha epitopos únicos que no son abordados por los medicamentos existentes, lo que podría superar los mecanismos de resistencia asociados con las terapias actuales dirigidas a HER2.
Tharimmune (NASDAQ:THAR)는 HS1940를 통해 제품 파이프라인을 확장했습니다. 이는 PD-1 및 VEGF 수용체를 동시에 표적하는 새로운 이중 표적 생물학적 제제로, 그들의 독점 기술인 EpiClick Technology를 사용합니다. HS1940은 PD-1 경로를 동시에 활성화하고 혈관 신생을 억제하도록 설계되어, 여러 종류의 암에 대한 개선된 치료 옵션을 제공할 수 있습니다.
회사는 이중 표적 형식이 단일 특이성 항체에 비해 향상된 치료의 다양성을 제공한다는 긍정적인 목표 참여 데이터를 생성했습니다. Tharimmune은 2025년 동안 HS1940에 대한 IND 승인 연구를 시작할 계획입니다.
또한, Tharimmune은 HER2 및 HER3 암 유발 인자를 표적하는 HS3215라는 다중 특이성 항체를 개발하고 있습니다. 이 접근 방식은 기존 약물로 해결되지 않은 독특한 에피토프를 활용하여 현재의 HER2 표적 치료와 관련된 저항 메커니즘을 극복할 수 있습니다.
Tharimmune (NASDAQ:THAR) a élargi son portefeuille de produits avec HS1940, un biologiquement biparatopique novateur ciblant à la fois les récepteurs PD-1 et VEGF en utilisant leur technologie propriétaire EpiClick Technology. HS1940 est conçu pour activer simultanément la voie PD-1 et inhiber l'angiogenèse, offrant potentiellement de meilleures options de traitement pour plusieurs types de cancer.
L'entreprise a généré des données positives sur l'engagement des cibles, montrant que le format biparatopique offre une polyvalence thérapeutique améliorée par rapport aux anticorps monospecifiques. Tharimmune prévoit de lancer des études d'habilitation IND pour HS1940 tout au long de 2025.
De plus, Tharimmune développe HS3215, un anticorps multispecifique ciblant les facteurs de cancer HER2 et HER3. Cette approche exploite des épitopes uniques non abordés par les médicaments existants, ce qui pourrait surmonter les mécanismes de résistance associés aux thérapies ciblées actuelles contre HER2.
Tharimmune (NASDAQ:THAR) hat sein Produktportfolio mit HS1940 erweitert, einem neuartigen biparatopischen Biologikum, das sowohl die PD-1- als auch die VEGF-Rezeptoren mit Hilfe ihrer proprietären EpiClick Technology anvisiert. HS1940 ist darauf ausgelegt, den PD-1-Weg gleichzeitig zu aktivieren und die Angiogenese zu hemmen, was potenziell verbesserte Behandlungsoptionen für verschiedene Krebsarten bieten könnte.
Das Unternehmen hat positive Daten zur Zielbindung generiert, die zeigen, dass das biparatopische Format eine verbesserte therapeutische Vielseitigkeit im Vergleich zu monospezifischen Antikörpern bietet. Tharimmune plant, im Laufe des Jahres 2025 IND-fähige Studien für HS1940 zu initiieren.
Darüber hinaus entwickelt Tharimmune HS3215, einen multispezifischen Antikörper, der die Krebsfaktoren HER2 und HER3 anvisiert. Dieser Ansatz nutzt einzigartige Epitope, die von bestehenden Medikamenten nicht angesprochen werden, und könnte so Resistenzmechanismen überwinden, die mit aktuellen HER2-zielgerichteten Therapien verbunden sind.
- Positive target engagement data achieved for HS1940
- Novel drug candidate targeting previously undruggable PD-1 epitopes
- Dual-action mechanism potentially offering improved cancer treatment efficacy
- Pipeline expansion with two promising candidates (HS1940 and HS3215)
- Products still in early development phase
- IND-enabling studies not yet initiated
- No clinical trial data available
Insights
Tharimmune's announcement reveals significant progress with their EpiClick™ technology platform, which enables development of novel multispecific biologics targeting previously undruggable epitopes. The positive target engagement data for HS1940, their biparatopic PD-1/VEGF biologic, represents a potentially important advancement in cancer immunotherapy.
What makes this approach scientifically noteworthy is the dual-pathway mechanism: simultaneously blocking PD-1 (releasing immune system "brakes") while inhibiting VEGF (disrupting tumor blood vessel formation). This combinatorial approach could potentially address limitations of existing single-target therapies, particularly in tumors that develop resistance.
The platform's ability to access novel epitopes on PD-1 that current market-leading inhibitors like nivolumab and pembrolizumab cannot reach represents a potential competitive advantage. Similarly, their HER2/HER3 multispecific program (HS3215) targets distinct epitopes that could potentially overcome resistance mechanisms seen with existing HER2 therapies.
However, investors should recognize these programs remain in preclinical development with IND-enabling studies for HS1940 planned throughout 2025, indicating a lengthy timeline before potential commercialization. While positive target engagement data is encouraging, it represents just the first step in a long development journey requiring substantial clinical validation.
Tharimmune's pipeline expansion with HS1940 and their EpiClick platform shows incremental technological progress but falls within standard R&D advancement for a clinical-stage biotech. The positive target engagement data, while promising, represents very early-stage preclinical validation with years of development ahead before potential commercialization.
The multispecific antibody approach targeting PD-1/VEGF and HER2/HER3 enters increasingly competitive therapeutic landscapes. The PD-1 inhibitor market is dominated by established players like Merck's Keytruda (
Tharimmune's differentiation lies in their unique epitope targeting and dual-pathway approach, which could potentially address resistance mechanisms. However, the company must still navigate extensive clinical trials, regulatory reviews, and demonstrate clinically meaningful differentiation to gain market share.
For a
BRIDGEWATER, NJ / ACCESS Newswire / March 4, 2025 / Tharimmune, Inc. (NASDAQ:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company focused on immunology and inflammation, today announced the expansion of its product pipeline with HS1940, a dual-target multispecific biologic engineered to bind to both Programmed Death-1 (PD-1) and Vascular Endothelial Growth Factor (VEGF) receptors. Using its proprietary EpiClick™ Technology, a versatile multispecific antibody engineering platform, HS1940 represents a key expansion of Tharimmune's product pipeline and underscores the Company's commitment to addressing unmet needs.
HS1940 is designed as a biparatopic (binding two epitopes on a single target) biologic, simultaneously engaging the PD-1 pathway and inhibiting angiogenesis. By using multiple, previously undruggable epitopes on PD-1, and blocking VEGF-mediated tumor vascularization, HS1940 may broaden treatment options and improve outcomes across multiple types of cancer and may access receptor regions that other PD-1 inhibitors (e.g., nivolumab and pembrolizumab) may not reach.
"We are extremely excited about the potential and versatility of our EpiClick platform. The implications for therapeutic intervention are potentially substantial, suggesting EpiClick-derived biologics may offer more potent and nuanced approaches to disease modulation," said Randy Milby, CEO of Tharimmune. "With EpiClick, we can strategically combine antibody binding domains to target multiple hard-to-reach epitopes, creating highly customized therapeutics tailored to the specifics of tumor destruction. For example, by addressing novel and multiple PD-1 epitopes while simultaneously inhibiting VEGF, we have the potential to offer a differentiated and more effective treatment option for a variety of solid tumors."
EpiClick enables the rapid and efficient creation of modular antibodies capable of high specificity and affinity toward multiple targets. A key feature of EpiClick is its "mix and match" approach, allowing distinct antibody binding domains - including those derived from previously inaccessible, undruggable epitopes - to be combined in either small-format or full-length configurations.
In the HS1940 family, the anti-PD-1 components leverage a novel design inspired by bovine antibodies, enabling them to target previously undruggable PD-1 epitopes. The anti-VEGF component, and the overall multispecific format, are engineered using EpiClick's modular design capabilities to create novel biparatopic PD-1 binding domains engineered on multiple VEGF scaffolds, as illustrated below.
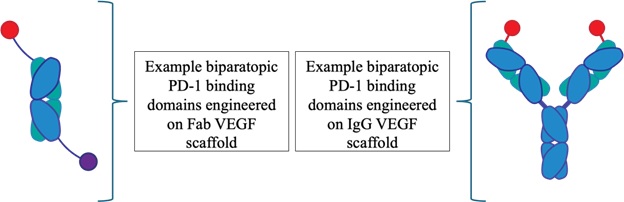
The Company has generated positive target engagement data showing that the biparatopic format expands therapeutic versatility compared with monospecific antibodies, which are limited to targeting a single epitope on a single receptor. As illustrated, the biparatopic antibody constructs effectively achieve target engagement on both PD-1 and VEGF. By engaging two distinct cellular pathways, HS1940 has the potential to elicit complex and synergistic anti-tumor effects. Tharimmune plans to continue preclinical testing with HS1940 and present data at future scientific conferences. The Company expects to initiate IND-enabling studies for HS1940 throughout 2025.
PD-1 is a well-validated immune checkpoint receptor that, when activated, suppresses T-cell function and allows cancer cells to evade immune detection. VEGF drives angiogenesis, which provides a nutrient and oxygen supply for tumors. By simultaneously blocking both pathways, HS1940 aims to achieve a synergistic anti-tumor effect by blocking PD-1, which releases immune "brakes," and enhancing T-cell-mediated tumor attack. Blocking VEGF disrupts tumor vasculature, starving them of nutrients.
Building upon the EpiClick platform, Tharimmune is also developing a new generation of multispecific antibodies targeting HER2 and HER3, two validated drivers of cancer growth and metastases. While HER2 is the focus of numerous successful commercial therapies, Tharimmune's approach offers distinct advantages. EpiClick leverages the "knob-and-stalk" from bovine-derived antibodies engineered to reach unique HER2 epitopes not addressed by existing drugs, while simultaneously engaging HER3. This dual engagement has the potential to disrupt cancer signaling in novel ways and overcome resistance to mechanisms associated with existing HER2-targeted therapies. By targeting distinct epitopes and incorporating HER3 engagement, Tharimmune's EpiClick-derived antibodies such as HS3215 offer a promising new avenue for more effective and targeted cancer treatments. Tharimmune is conducting preclinical studies to evaluate and optimize HS3215, with plans to advance the molecule into clinical trials following IND-enabling studies.
About EpiClick™ Technology
EpiClick™ Technology is a platform for creating customizable, multispecific antibodies that target previously undruggable epitopes, including those on PD-1, HER2, HER3 and other validated cancer targets. Inspired by bovine antibodies' unique "knob-and-stalk" structure, EpiClick uses engineered "knob" domains - small, precise binding units - that can "click" into recessed protein sites inaccessible to conventional antibodies. Its modular nature allows these "knobs," each targeting a specific epitope, to be paired with additional antibody components, creating a vast combinatorial library of multispecific therapeutics. For example, EpiClick enables the creation of novel biparatopic anti-PD-1 components, as used in HS1940, or HER2/HER3 domains, as in HS3215. By unlocking undruggable epitopes, EpiClick aims to deliver more effective and targeted treatments in immunotherapy and cancer therapy.
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts
Tharimmune, Inc.
ir@tharimmune.com
Alliance Advisors IR
Tirth T. Patel
tpatel@allianceadvisors.com
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire







