Tharimmune Announces Positive Results for Novel Oral Monoclonal Antibody TH023 Targeting Tumor Necrosis Factor-alpha
Tharimmune (NASDAQ:THAR) has announced positive preclinical results for its novel oral antibody TH023, showing successful delivery of infliximab, a TNF-α inhibitor, in serum with concentrations higher than standard trough levels needed for efficacy (~3-5μg/ml).
The study demonstrated enzymatic protection of infliximab against human colon enzymes using the Soteria® platform, and successful delivery both into colonic tissue and systemic circulation following intra-duodenal dosing in mice. The company's proprietary formulation outperformed traditional permeation enhancers like SNAC in tissue penetration.
TH023 represents a potential breakthrough in converting injectable infliximab into an oral format, targeting both local gastrointestinal diseases like IBD and systemic inflammatory conditions. The global infliximab market, valued at $6.3 billion in 2022, could reach $9 billion within 10 years. Tharimmune plans to initiate first-in-human clinical trials within the next 12 months.
Tharimmune (NASDAQ:THAR) ha annunciato risultati preclinici positivi per il suo nuovo anticorpo orale TH023, mostrando una consegna efficace di infliximab, un inibitore del TNF-α, nel siero con concentrazioni superiori ai livelli minimi standard necessari per l'efficacia (~3-5μg/ml).
Lo studio ha dimostrato la protezione enzimatica di infliximab contro gli enzimi del colon umano utilizzando la piattaforma Soteria®, e una consegna efficace sia nei tessuti colici che nella circolazione sistemica dopo somministrazione intra-duodenale nei topi. La formulazione proprietaria dell'azienda ha superato gli tradizionali potenziatori di permeazione come SNAC nella penetrazione tissutale.
TH023 rappresenta una potenziale svolta nella conversione dell'infliximab iniettabile in un formato orale, mirando a malattie gastrointestinali locali come la IBD e condizioni infiammatorie sistemiche. Il mercato globale dell'infliximab, valutato 6,3 miliardi di dollari nel 2022, potrebbe raggiungere i 9 miliardi di dollari entro 10 anni. Tharimmune prevede di avviare i primi studi clinici sull'uomo entro i prossimi 12 mesi.
Tharimmune (NASDAQ:THAR) ha anunciado resultados preclínicos positivos para su novedoso anticuerpo oral TH023, mostrando una entrega exitosa de infliximab, un inhibidor del TNF-α, en suero con concentraciones superiores a los niveles mínimos estándar necesarios para la eficacia (~3-5μg/ml).
El estudio demostró la protección enzimática de infliximab contra las enzimas del colon humano utilizando la plataforma Soteria®, y una entrega exitosa tanto en el tejido colónico como en la circulación sistémica tras la dosificación intra-duodenal en ratones. La formulación propietaria de la empresa superó a los potenciadores de permeación tradicionales como SNAC en la penetración del tejido.
TH023 representa un posible avance en la conversión de infliximab inyectable en un formato oral, dirigido tanto a enfermedades gastrointestinales locales como la EII y condiciones inflamatorias sistémicas. El mercado global de infliximab, valorado en 6.3 mil millones de dólares en 2022, podría alcanzar los 9 mil millones de dólares en 10 años. Tharimmune planea iniciar ensayos clínicos en humanos en los próximos 12 meses.
Tharimmune (NASDAQ:THAR)는 새로운 경구 항체 TH023에 대한 긍정적인 전임상 결과를 발표하며, TNF-α 억제제인 인플릭시맙의 성공적인 전달이 효능을 위해 필요한 표준 최저 수준(~3-5μg/ml)보다 높은 혈청 농도로 이루어졌음을 보여주었습니다.
이 연구는 Soteria® 플랫폼을 사용하여 인플릭시맙이 인간 대장 효소에 대해 효소적 보호를 받았음을 입증했으며, 마우스에서 내십이지장 투여 후 대장 조직과 전신 순환으로의 성공적인 전달을 보여주었습니다. 회사의 독점 제형은 SNAC과 같은 전통적인 투과 증진제보다 조직 침투에서 뛰어난 성능을 보였습니다.
TH023은 주사 가능한 인플릭시맙을 경구 형식으로 전환하는 잠재적 돌파구를 나타내며, IBD와 같은 국소 위장 질환 및 전신 염증 상태를 목표로 하고 있습니다. 2022년 63억 달러로 평가된 글로벌 인플릭시맙 시장은 10년 내에 90억 달러에 이를 수 있습니다. Tharimmune은 향후 12개월 내에 인간 대상의 첫 번째 임상 시험을 시작할 계획입니다.
Tharimmune (NASDAQ:THAR) a annoncé des résultats précliniques positifs pour son nouvel anticorps oral TH023, montrant une livraison réussie d'infliximab, un inhibiteur du TNF-α, dans le sérum avec des concentrations supérieures aux niveaux minimaux standard nécessaires à l'efficacité (~3-5μg/ml).
Cette étude a démontré la protection enzymatique de l'infliximab contre les enzymes coliques humaines en utilisant la plateforme Soteria®, et une livraison réussie à la fois dans les tissus coliques et dans la circulation systémique après administration intra-duodénale chez des souris. La formulation propriétaire de l'entreprise a surpassé les agents de perméation traditionnels tels que le SNAC en matière de pénétration tissulaire.
TH023 représente une avancée potentielle dans la conversion de l'infliximab injectable en un format oral, ciblant à la fois les maladies gastro-intestinales locales comme la MII et les conditions inflammatoires systémiques. Le marché mondial de l'infliximab, évalué à 6,3 milliards de dollars en 2022, pourrait atteindre 9 milliards de dollars d'ici 10 ans. Tharimmune prévoit de lancer les premiers essais cliniques chez l'homme dans les 12 prochains mois.
Tharimmune (NASDAQ:THAR) hat positive präklinische Ergebnisse für seinen neuartigen oralen Antikörper TH023 bekannt gegeben, der eine erfolgreiche Abgabe von Infliximab, einem TNF-α-Hemmer, im Serum mit Konzentrationen zeigt, die über den erforderlichen Standardminimumwerten für die Wirksamkeit liegen (~3-5μg/ml).
Die Studie demonstrierte den enzymatischen Schutz von Infliximab gegen menschliche Dickdarm-Enzyme unter Verwendung der Soteria®-Plattform und eine erfolgreiche Abgabe sowohl in das Dickdarmgewebe als auch in die systemische Zirkulation nach intra-duodenaler Verabreichung bei Mäusen. Die proprietäre Formulierung des Unternehmens übertraf traditionelle Permeationsverstärker wie SNAC bei der Gewebepenetration.
TH023 stellt einen potenziellen Durchbruch dar, um injizierbares Infliximab in ein orales Format umzuwandeln, das sowohl lokale gastrointestinalen Erkrankungen wie IBD als auch systemische entzündliche Erkrankungen anspricht. Der globale Infliximab-Markt, der 2022 auf 6,3 Milliarden Dollar geschätzt wurde, könnte in 10 Jahren 9 Milliarden Dollar erreichen. Tharimmune plant, innerhalb der nächsten 12 Monate die ersten klinischen Studien am Menschen zu starten.
- None.
- None.
Insights
Tharimmune's preclinical results for TH023 represent a potentially significant technological breakthrough in biologics delivery. The data demonstrating successful oral delivery of infliximab with serum concentrations exceeding the therapeutic threshold (
The market opportunity is substantial. Infliximab products generated approximately
What's particularly compelling is the comparative data showing superiority over established permeation enhancers like SNAC (used in semaglutide formulations), suggesting a competitive moat in oral antibody delivery. The validation of both local GI and systemic delivery creates potential for addressing multiple indications beyond just IBD.
However, investors should temper expectations with timeline realities. The 12-month horizon to first-in-human trials signals we're still years from commercialization, and success in murine models frequently fails to translate to humans for complex biologics. The preclinical nature of this development means substantial technical, regulatory, and commercial hurdles remain before TH023 could become a viable product.
The preclinical data for TH023 demonstrates a compelling proof-of-concept for oral antibody delivery, addressing a fundamental pharmaceutical challenge. The dual mechanism leveraging both passive transcytosis and active FcRn-mediated transport represents sophisticated pharmacokinetic engineering with several key advantages over current approaches.
First, achieving therapeutic serum concentrations exceeding the
Second, the dual-action pharmacokinetic profile showing both local colonic tissue penetration and systemic circulation creates versatility that's ideal for inflammatory conditions. For IBD specifically, local delivery directly to affected tissues could potentially improve efficacy while reducing systemic exposure-related adverse events.
The comparative advantage over established permeation enhancers like SNAC, sodium caprate, and labrasol further validates their approach, suggesting that standard peptide delivery technologies are indeed unsuitable for large antibody molecules. The Soteria® platform's ability to protect infliximab against colon enzymes addresses a critical limitation of previous oral biologic delivery attempts.
While these results are promising, the translation from murine models to human subjects remains the critical challenge ahead, particularly regarding bioavailability consistency, dosing optimization, and immunogenicity considerations that can only be fully assessed in clinical trials.
BRIDGEWATER, NJ / ACCESS Newswire / March 24, 2025 / Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company focused on immunology and inflammation, today announced positive preclinical results for its novel oral antibody, TH023. In a murine model, a proprietary protease enzyme stabilized platform demonstrated successful delivery of infliximab, a tumor necrosis factor-alpha (TNF-α) inhibitor, in serum with concentrations detected being significantly higher than the standard serum trough concentration needed for antibody efficacy in immunology indications via injection (~3-5µg/ml). These findings represent a significant step towards developing a more convenient and potentially patient-preferred alternative to currently available infliximab treatments, which are administered via intravenous infusion or subcutaneous injection.
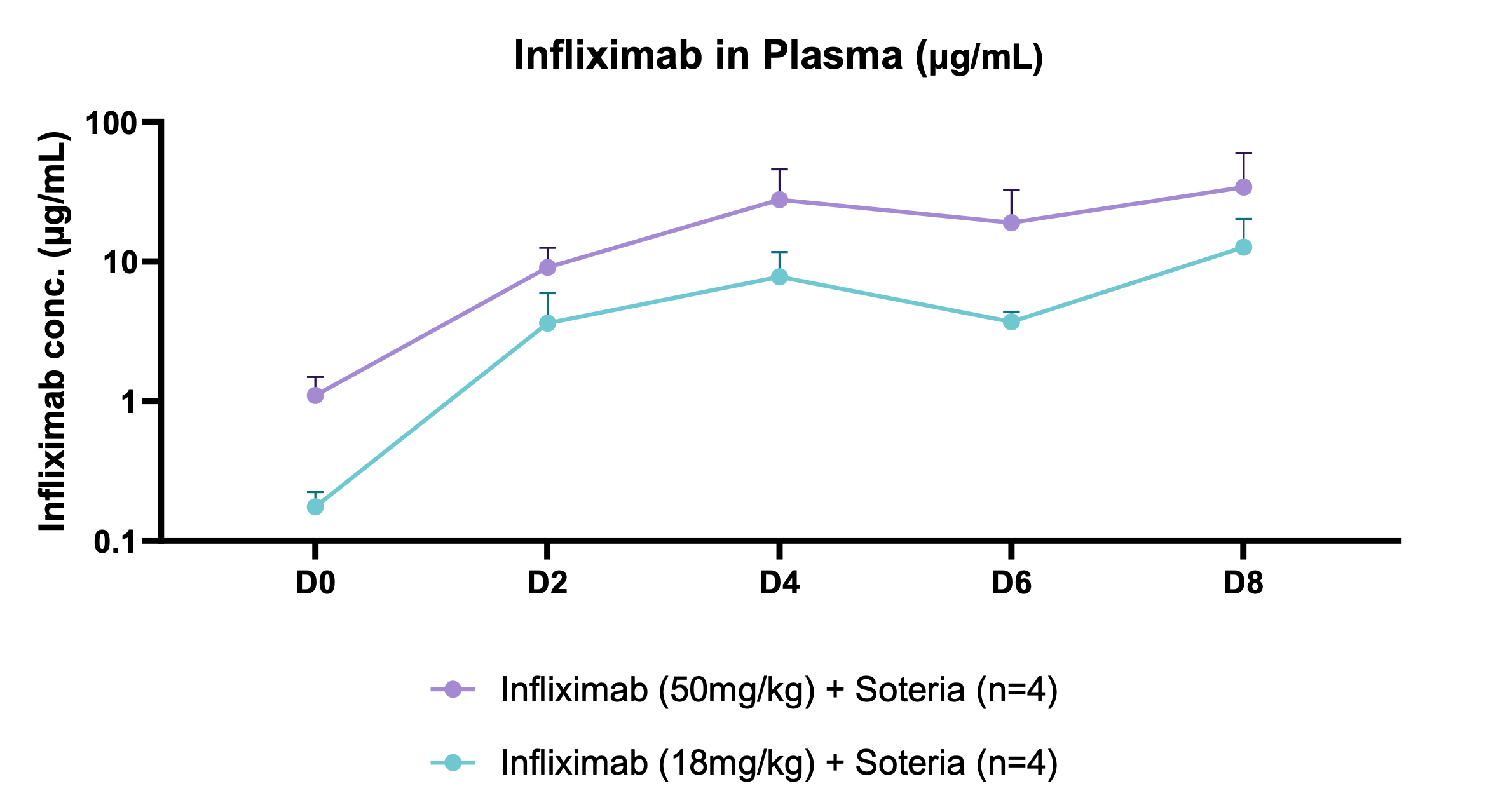
Key findings of the preclinical evaluation include demonstrating enzymatic protection of infliximab against human colon enzymes ex vivo using fresh fecal samples from healthy subjects utilizing the Soteria® platform, a proprietary formulation of natural amino acids (data not shown). Furthermore, successful delivery of TH023 in vivo into both local colonic tissue and systemic circulation was shown following intra-duodenal once-daily dosing for 1 week in a healthy mouse model at two doses of infliximab. This data shows the potential of the delivery platform to allow for both local delivery of the antibody precisely in the large intestinal tissue through enzymatic stabilization, as well as systemic circulation, which is an ideal pharmacokinetic (PK) profile for targeting both local gastrointestinal (GI) diseases such as inflammatory bowel disease (IBD) as well as systemic inflammatory diseases. The mechanism by which the antibody transcytosis occurs in the GI tract was shown to be a combination of passive, as well as mediated via the neonatal fragment crystallizable receptor (FcRn), highly expressed in distal intestinal epithelial cells enabling active transport.
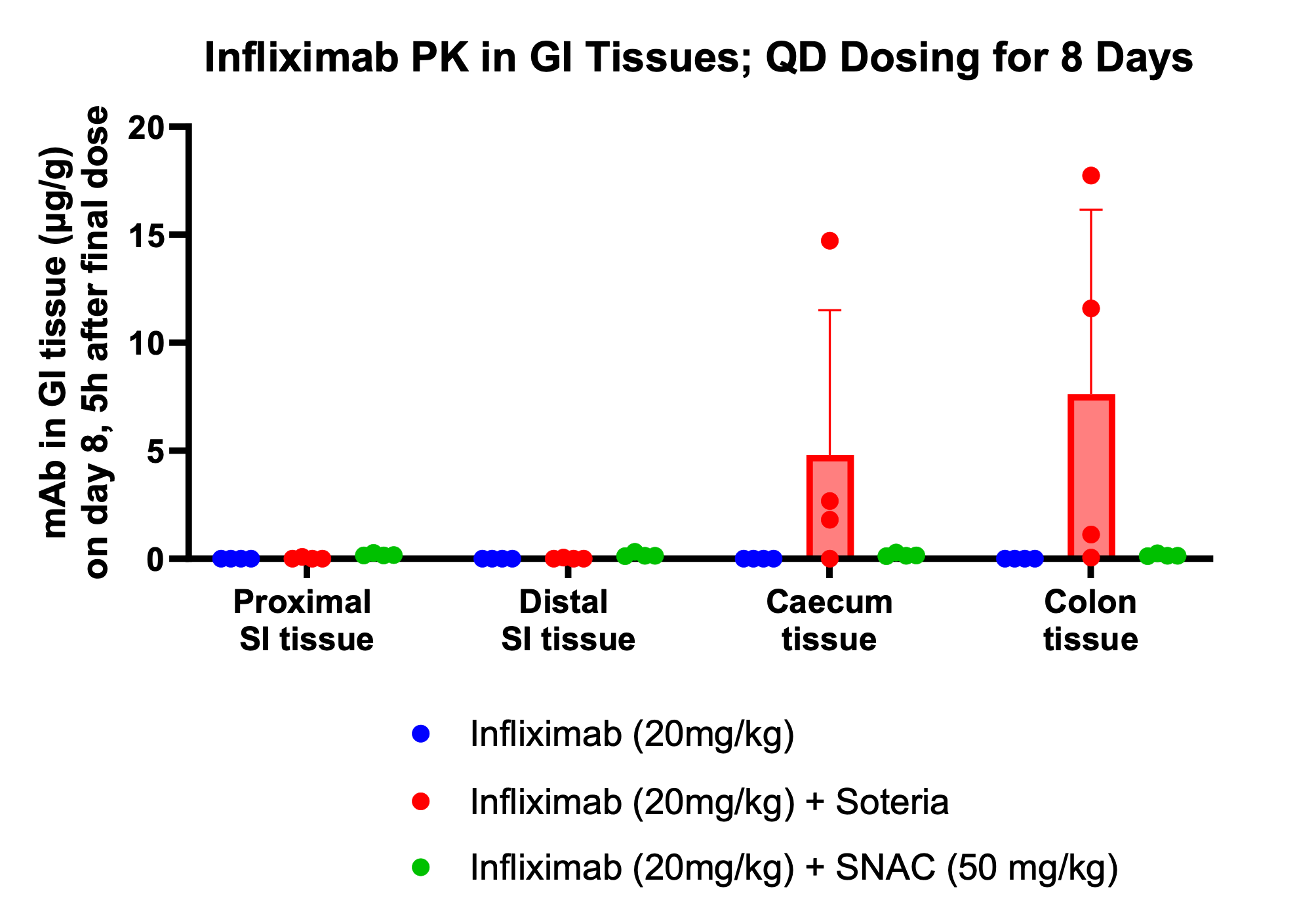
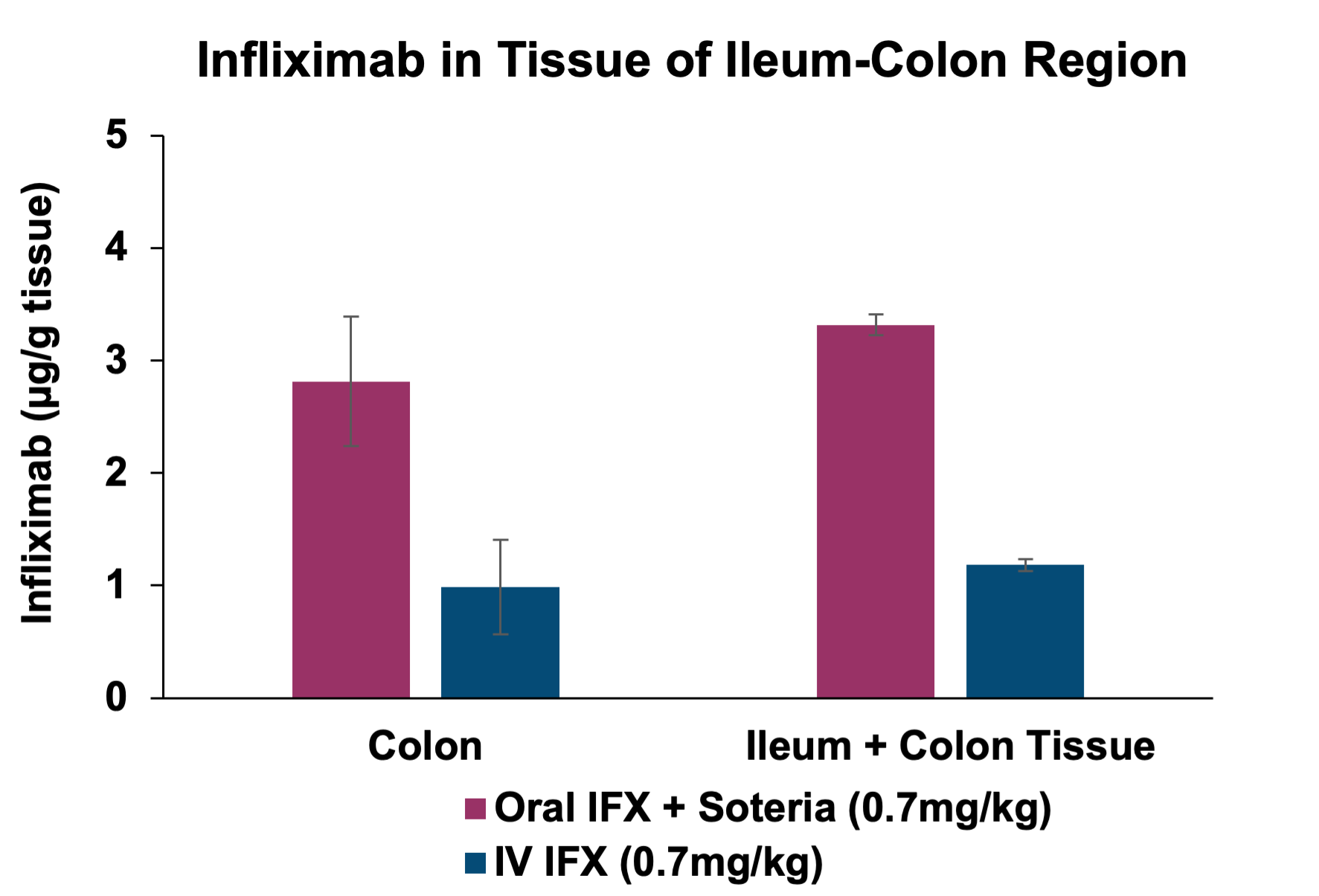
Additionally, the study demonstrated that tissue penetration of infliximab in combination with the enzyme stabilization platform was superior to a traditional permeation enhancer, sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate (SNAC), which has been used to enhance the absorption of GLP-1 peptides, such as semaglutide. Utilization of SNAC to protect infliximab from enzymatic degradation or permeation enhancement did not result in tissue or serum concentrations suggesting standard off-the-shelf oral peptide delivery technologies are not suitable for oral delivery of antibodies. Two other standard permeation enhancer technologies tested (sodium caprate and labrasol) also showed unsitable results for oral delivery (data not shown), further supporting the Company's proprietary TH023 formulation.
The Company announced last year through a partnership with Intract Pharma, an exclusive license to INT-023 (now TH023), an oral anti-TNF-α monoclonal antibody. Tharimmune licensed global development and commercialization rights (outside of South Korea) to Intract Pharma's Soteria® and Phloral® delivery platform along with an existing supply agreement for infliximab to be used in the oral product development program. Traditionally administered through intravenous infusions, oral delivery of antibodies is challenging due to the complexity of navigating such large molecules through the GI tract. An oral route of administration holds potential to improve patient compliance and quality of life, while also reducing the burden on the healthcare system associated with long-term intravenous therapy.
"We are extremely encouraged by these results, which validate the potential of our partnership with Intract and the platform to deliver complex biologic molecules like infliximab orally," said Randy Milby, CEO of Tharimmune. "This represents a potential major milestone in our mission to develop more patient-friendly and accessible treatment options for chronic inflammatory diseases, addressing a multi-billion dollar market. These findings provide a strong foundation for further development into clinical trials."
Through the Company's existing partnership with Intract the data announced today enables for the targeted delivery of antibody therapeutics directly to the colon or small intestine. By leveraging Intract's platform, Tharimmune aims to enhance the effectiveness of TNF-α inhibitors such as infliximab through precision delivery that maximizes proteolytic stabilization and tissue permeation. This novel approach offers significant potential for directly addressing inflammatory conditions within the GI tract, including IBD as well as systemic inflammatory disorders where TNF-α plays a critical role in disease progression. Tharimmune plans to optimize the formulation and dosing regimen and prepare to conduct a first-in-human clinical trial with TH023 in the next 12 months.
Infliximab, a TNF-α inhibitor, is a widely used biologic for the treatment of several chronic inflammatory diseases, including Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. Globally, infliximab (including biosimilars) generated approximately
About Tharimmune, Inc.
Tharimmune is a clinical-stage biotechnology company developing a diverse portfolio of therapeutic candidates in immunology, inflammation and oncology. Its lead clinical asset, TH104, aims to suppress chronic pruritus associated with primary biliary cholangitis (PBC), a rare autoimmune liver disease with no known cure. The expanded pipeline includes TH023, an oral TNF-alpha inhibitor offering a new approach to treating autoimmune diseases. Tharimmune is also advancing early-stage multispecific biologics targeting unique epitopes against multiple solid tumors through its proprietary EpiClick™ Technology. The company has a license agreement with OmniAb, Inc. to access their antibody discovery technology for targeting specified disease markers. For more information, please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding the timing and design of Tharimmune's future Phase 2 trial, Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
Contacts:
Tharimmune, Inc.
ir@tharimmune.com
Alliance Advisors IR
Tirth T. Patel
tpatel@allianceadvisors.com
212-201-6614
SOURCE: Tharimmune Inc.
View the original press release on ACCESS Newswire






