Stratasys Launches TrueDent in Europe, Bringing the Benefits of Monolithic Digital Dentures to Dental Labs, Dentists, and Patients
TrueDent-D™ resin is now available in major European markets that require CE marking, with strong customer interest already building
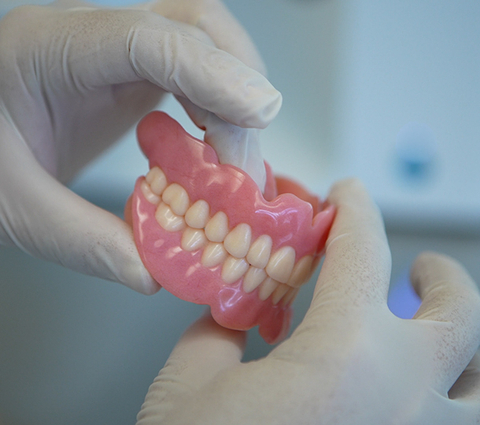
Stratasys Launches TrueDent™ in
Following its successful debut in
According to a recent iData report1, the demand for denture solutions in
A key feature is the ability to print a duplicate denture with a click of a button enabling clinicians to quickly provide a spare or a backup for their patients. TrueDent is the only solution in the world that can produce identical dentures when needed, offering not just fit, form and function, but also aesthetics. This capability creates potential for new business models, allowing customers to scale production without increasing costs or labor requirements.
“The TrueDent solution has transformed our denture business,” said Tra’ Chambers, Owner of Express Dental Laboratories, a leading
Edentulism, the condition of being without natural teeth, affects over 267.5 million people globally, including a prevalence rate exceeding
The TrueDent denture solution elevates the challenges faced by labs that are limited by current fabrication methods that often involve the need for manual assembly by skilled craftspeople. TrueDent is already proving its value in the
“We are thrilled to bring TrueDent-D to Europe,” said Erez Ben Zvi, Vice President of Healthcare at Stratasys. “Our monolithic TrueDent denture solution combines high fidelity, aesthetics, and production scalability, while reducing labor costs and enabling exact reproductions. There is growing excitement across the region for this innovative solution, which not only improves the experience for dental professionals but also elevates the standard of care for patients.”
For more information about the TrueDent digital denture application, visit the Stratasys TrueDent page.
1 – iData Europe Market Report Suite for Dental Prosthetics, February 2024
About Stratasys
Stratasys is leading the global shift to additive manufacturing with innovative 3D printing solutions for industries such as aerospace, automotive, consumer products, and healthcare. Through smart and connected 3D printers, polymer materials, a software ecosystem, and parts on demand, Stratasys solutions deliver competitive advantages at every stage in the product value chain. The world’s leading organizations turn to Stratasys to transform product design, bring agility to manufacturing and supply chains, and improve patient care.
To learn more about Stratasys, visit www.stratasys.com, the Stratasys blog, X/Twitter, LinkedIn, or Facebook. Stratasys reserves the right to utilize any of the foregoing social media platforms, including Stratasys’ websites, to share material, non-public information pursuant to the SEC’s Regulation FD. To the extent necessary and mandated by applicable law, Stratasys will also include such information in its public disclosure filings.
Stratasys, TrueDent, TrueDent-D and J5 DentaJet are trademarks or registered trademarks of Stratasys Ltd. and/or its affiliates. All other trademarks are the property of their respective owners.
Note Regarding Forward-Looking Statement
The statements in this press release relating to Stratasys’ beliefs regarding the benefits consumers will experience from using the TrueDent-D resin, it’s time of general ability and other statements in this press release are forward-looking statements reflecting management's current expectations and beliefs. These forward-looking statements are based on current information that is, by its nature, subject to rapid and even abrupt change. Due to risks and uncertainties associated with Stratasys' business, actual results could differ materially from those projected or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to: the degree of our success at introducing new or improved products and solutions that gain market share; the degree of growth of the 3D printing market generally; the impact of potential shifts in the prices or margins of the products that we sell or services that we provide, including due to a shift towards lower-margin products or services; the impact of competition and new technologies; potential further charges against earnings that we could be required to take due to impairment of additional goodwill or other intangible assets; to the extent of our success at successfully consummating acquisitions or investments in new businesses, technologies, products or services; potential changes in our management and board of directors; global market, political and economic conditions, and in the countries in which we operate in particular; risks related to infringement of our intellectual property rights by others or infringement of others' intellectual property rights by us; the extent of our success at maintaining our liquidity and financing our operations and capital needs; the impact of tax regulations on our results of operations and financial condition; and other risk factors set forth under the caption “Risk Factors” in Stratasys’ most recent Annual Report on Form 20-F, filed with the Securities and Exchange Commission (SEC) on March 11th, 2024. Readers are urged to carefully review and consider the various disclosures made throughout our 2023 Annual Report and our other reports filed with or furnished to the SEC, which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects. Any guidance provided, and other forward-looking statements made, in this press release are made as of the date hereof, and Stratasys undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250114856677/en/
Media and Investor contacts:
Stratasys Corporate,
Chris Reese
chris.reese@stratasys.com
+1 651 357 0877
Stratasys Corporate,
Erik Snider
Erik.Snider@stratasys.com
+972 74 745 6053
Investor Relations
Yonah Lloyd
Yonah.Lloyd@stratasys.com
+972 74 745 4919
Source: Stratasys









