Rani Therapeutics Announces Preclinical Data Demonstrating Bioequivalence of RT-114, a GLP-1/GLP-2 Dual Agonist (PG-102) Delivered Orally via the RaniPill® Capsule, to Subcutaneously Administered PG-102
Rani Therapeutics (RANI) announced promising preclinical data for RT-114, their oral GLP-1/GLP-2 dual agonist delivered via RaniPill® capsule. The study demonstrated bioequivalence with subcutaneously administered PG-102, achieving 111% relative bioavailability and comparable pharmacokinetic profiles.
Key findings from the canine study include:
- 90% delivery success rate (9 out of 10 canines)
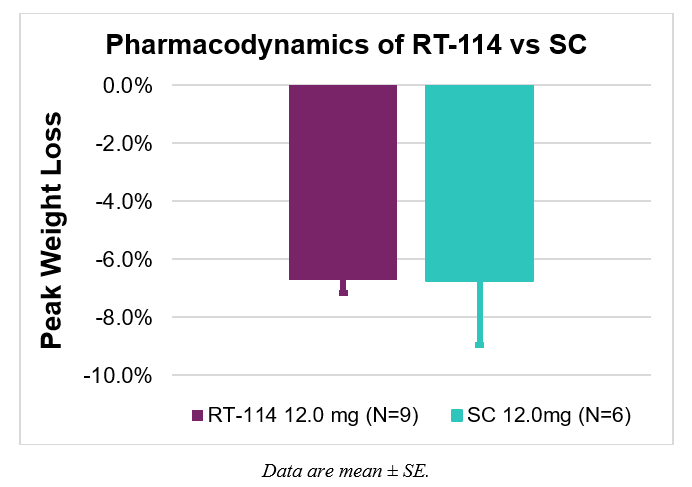
- Identical average peak weight loss of 6.7% in both oral and subcutaneous groups
- Well-tolerated with no safety concerns
Previous Phase 1 trials of subcutaneous PG-102 showed weight loss in obese patients, averaging 4.8% and reaching up to 8.7% after five weeks. The company plans to initiate a Phase 1 clinical trial for RT-114 in mid-2025, targeting obesity treatment with a convenient oral dosing regimen.
Rani Therapeutics (RANI) ha annunciato dati preclinici promettenti per RT-114, il loro agonista duale GLP-1/GLP-2 somministrato tramite capsula RaniPill®. Lo studio ha dimostrato bioequivalenza con il PG-102 somministrato per via sottocutanea, raggiungendo una biodisponibilità relativa del 111% e profili farmacocinetici comparabili.
I principali risultati dello studio sui cani includono:
- 90% di successo nella somministrazione (9 su 10 cani)
- Identica perdita media di peso massima del 6,7% in entrambi i gruppi, orale e sottocutaneo
- Ben tollerato, senza preoccupazioni di sicurezza
I precedenti studi di Fase 1 sul PG-102 sottocutaneo hanno mostrato una perdita di peso nei pazienti obesi, con una media del 4,8% e un massimo dell'8,7% dopo cinque settimane. L'azienda prevede di avviare un trial clinico di Fase 1 per RT-114 a metà del 2025, mirato al trattamento dell'obesità con un regime di dosaggio orale conveniente.
Rani Therapeutics (RANI) anunció datos preclínicos prometedores para RT-114, su agonista dual GLP-1/GLP-2 administrado a través de la cápsula RaniPill®. El estudio demostró bioequivalencia con el PG-102 administrado por vía subcutánea, logrando una biodisponibilidad relativa del 111% y perfiles farmacocinéticos comparables.
Los hallazgos clave del estudio en caninos incluyen:
- Tasa de éxito de entrega del 90% (9 de 10 caninos)
- Pérdida de peso pico promedio idéntica del 6.7% en ambos grupos, oral y subcutáneo
- Bien tolerado, sin preocupaciones de seguridad
Los ensayos de Fase 1 previos del PG-102 subcutáneo mostraron pérdida de peso en pacientes obesos, promediando un 4.8% y alcanzando hasta un 8.7% después de cinco semanas. La empresa planea iniciar un ensayo clínico de Fase 1 para RT-114 a mediados de 2025, enfocado en el tratamiento de la obesidad con un régimen de dosificación oral conveniente.
Rani Therapeutics (RANI)는 RT-114에 대한 유망한 전임상 데이터를 발표했습니다. 이는 RaniPill® 캡슐을 통해 전달되는 경구 GLP-1/GLP-2 이중 작용제입니다. 이 연구는 피하 투여된 PG-102와의 생물학적 동등성을 입증하며, 111%의 상대 생체이용률과 유사한 약리학적 프로파일을 달성했습니다.
개 연구의 주요 결과는 다음과 같습니다:
- 90%의 전달 성공률 (10마리 중 9마리)
- 경구 및 피하 그룹 모두에서 동일한 평균 최대 체중 감소율 6.7%
- 안전성 문제 없이 잘 견딜 수 있음
이전의 1상 임상 시험에서 피하 PG-102는 비만 환자에서 평균 4.8%의 체중 감소를 보였으며, 5주 후 최대 8.7%에 도달했습니다. 회사는 2025년 중반에 RT-114에 대한 1상 임상 시험을 시작할 계획이며, 편리한 경구 용법으로 비만 치료를 목표로 하고 있습니다.
Rani Therapeutics (RANI) a annoncé des données précliniques prometteuses pour RT-114, leur agoniste dual GLP-1/GLP-2 administré par le biais de la capsule RaniPill®. L'étude a démontré une bioéquivalence avec le PG-102 administré par voie sous-cutanée, atteignant une biodisponibilité relative de 111% et des profils pharmacocinétiques comparables.
Les principales conclusions de l'étude canine incluent:
- Taux de réussite de livraison de 90% (9 sur 10 chiens)
- Perte de poids maximale moyenne identique de 6,7% dans les deux groupes, oral et sous-cutané
- Bien toléré, sans préoccupations de sécurité
Les essais de Phase 1 précédents sur le PG-102 sous-cutané ont montré une perte de poids chez des patients obèses, avec une moyenne de 4,8% et atteignant jusqu'à 8,7% après cinq semaines. L'entreprise prévoit de lancer un essai clinique de Phase 1 pour RT-114 à la mi-2025, visant à traiter l'obésité avec un schéma posologique oral pratique.
Rani Therapeutics (RANI) hat vielversprechende präklinische Daten für RT-114 veröffentlicht, ihren oralen GLP-1/GLP-2-Dualagonisten, der über die RaniPill®-Kapsel verabreicht wird. Die Studie zeigte Bioäquivalenz zu subkutan verabreichtem PG-102, mit einer relativen Bioverfügbarkeit von 111% und vergleichbaren pharmakokinetischen Profilen.
Wichtige Ergebnisse der Hundestudie umfassen:
- 90% Erfolgsquote bei der Verabreichung (9 von 10 Hunden)
- Identischer durchschnittlicher maximaler Gewichtsverlust von 6,7% in beiden Gruppen, oral und subkutan
- Gut verträglich, ohne Sicherheitsbedenken
Frühere Phase-1-Studien zu subkutanem PG-102 zeigten einen Gewichtsverlust bei fettleibigen Patienten, im Durchschnitt 4,8% und bis zu 8,7% nach fünf Wochen. Das Unternehmen plant, Mitte 2025 eine Phase-1-Studie für RT-114 zu starten, die sich auf die Behandlung von Fettleibigkeit mit einem praktischen oralen Dosierungsschema konzentriert.
- Achieved 111% bioavailability compared to subcutaneous delivery
- 90% delivery success rate in preclinical trials
- Demonstrated comparable weight loss to injectable version with less variability
- No safety concerns or treatment discontinuations observed
- Potential for faster onset of effect with shorter titration schedule
- Phase 1 clinical trials not starting until mid-2025
- Currently only preclinical data available for oral delivery method
Insights
Rani Therapeutics' preclinical data for RT-114 represents a significant technical achievement in the oral biologics delivery space. The RaniPill capsule demonstrated 111% relative bioavailability compared to subcutaneous injection of PG-102, a GLP-1/GLP-2 dual agonist - meeting the critical bioequivalence endpoint with comparable pharmacokinetic profiles.
The weight loss data is particularly compelling, with RT-114 showing the same average peak weight loss (6.7%) as subcutaneous administration but with notably less variability (±0.5% vs ±2.2%), suggesting potentially more predictable clinical outcomes. The successful delivery rate of 90% indicates good reliability for a novel delivery technology at this stage.
The dual GLP-1/GLP-2 agonist mechanism offers key differentiation from single-target GLP-1 drugs currently dominating the obesity market. PG-102's early human data showing 4.8% average weight reduction (up to 8.7%) after just five weeks of subcutaneous dosing is promising, particularly with its apparently favorable tolerability profile allowing rapid dose escalation.
With RT-114 representing the fourth incretin successfully delivered via RaniPill (following semaglutide), the platform's versatility appears increasingly validated. However, investors should note that human testing won't begin until mid-2025, placing any potential commercialization several years away.
This preclinical milestone carries substantial strategic value for Rani Therapeutics by demonstrating the potential to solve the injectable delivery problem for obesity medications - one of the most significant growth markets in pharmaceuticals. The RaniPill platform continues showing consistent results across multiple incretin molecules, enhancing platform validation and potential partnership value.
RT-114's differentiated mechanism as a GLP-1/GLP-2 dual agonist could address a key unmet need in the obesity market: improved body composition during weight loss. The preclinical data showing equivalent weight loss to injected administration with less variability suggests potential for a best-in-class oral obesity medication if these results translate to humans.
The accelerated dose titration schedule seen with subcutaneous PG-102 (reaching target dose within one month) could address a major limitation of current GLP-1 therapies, which typically require 4-5 months before meaningful weight loss occurs. This faster onset combined with oral delivery would significantly enhance the commercial profile.
Given Rani's
- RT-114 yielded a relative bioavailability of
- Both groups demonstrated comparable weight loss with less variability observed with RT-114 compared to subcutaneous PG-102 –
- Phase 1 clinical trial of subcutaneous PG-102 demonstrated weight loss in obese patients, with an average reduction of
- Data bolster expanding body of evidence of the RaniPill® platform’s potential to facilitate oral delivery of multiple obesity treatments including previously announced semaglutide -
- Phase 1 clinical study for RT-114 for the treatment of obesity expected to initiate in mid- 2025 -
SAN JOSE, Calif., March 26, 2025 (GLOBE NEWSWIRE) -- Rani Therapeutics Holdings, Inc. (“Rani Therapeutics” or “Rani”) (Nasdaq: RANI), a clinical-stage biotherapeutics company focused on the oral delivery of biologics and drugs, today announced pharmacokinetic and pharmacodynamic data from a preclinical study evaluating RT-114, a GLP-1/GLP-2 dual agonist (PG-102). PG-102 delivered orally via the RaniPill® capsule demonstrated comparable bioavailability and weight loss to subcutaneously (SC) injected PG-102 (“SC PG-102”). PG-102 is ProGen Co., Ltd’s (“ProGen”) Fc-fusion protein conjugated GLP-1/GLP-2 dual agonist.
“We believe that RT-114 has the potential to be a first-in-class, orally administered GLP-1/GLP-2 dual agonist for the treatment of obesity, addressing a critical gap in the treatment landscape. Despite the remarkable success of GLP-1 receptor agonists, there is a pressing need for effective oral therapies with convenient dosing strategies to eliminate the need for burdensome injections. With RT-114’s extended half-life, we are targeting a convenient, oral dosing regimen for the treatment of obesity,” said Talat Imran, Chief Executive Officer of Rani Therapeutics. “Furthermore, in our preclinical study, RT-114 achieved pharmacokinetics, bioavailability, and weight loss comparable to PG-102 delivered via subcutaneous injection. We believe that PG-102’s GLP-1/GLP-2 dual agonist construct is key to its potential to induce higher quality weight loss, positioning RT-114 to potentially deliver improved body composition and nutritional health as an oral therapy. We are encouraged by the data generated to date and look forward to advancing RT-114 into a Phase 1 clinical trial this year.”
RT-114 represents the fourth incretin-based drug to be studied preclinically in the RaniPill® capsule or via the RaniPill® route of delivery. In February 2025, Rani announced preclinical data of semaglutide delivered via the RaniPill® capsule.
ProGen recently announced preliminary results of the repeat-dose portion (Phase 1C) of its Phase 1 study where SC PG-102 demonstrated weight loss in obese subjects, with an average reduction of
“First with semaglutide and now with RT-114, Rani has demonstrated comparable pharmacokinetics and weight loss outcomes in an oral delivery at the same dose as their respective injectable counterparts. This achievement is unprecedented when compared to existing oral GLP therapies on the market and in development," said Jesper Høiland, Senior Strategic Advisor at Rani Therapeutics and former President & EVP, USA at Novo Nordisk. "Furthermore, the short titration schedule and promising tolerability profile observed with subcutaneous PG-102 in the Phase 1C study may facilitate a quicker onset of effect. This could address a significant challenge with current GLP-1 treatment options, where patients typically do not experience clinically meaningful weight loss until after 4 to 5 months of treatment. By combining these potential advantages of PG-102 with the convenience of an oral delivery, Rani has the opportunity to create a truly differentiated product profile with RT-114.”
RT-114 Study Design
- The preclinical study was a head-to-head comparison of orally administered PG-102 via RaniPill® capsule (RT-114) and subcutaneously administered PG-102 in 16 healthy canines.
- All canines received a 12mg dose of PG-102 either via RaniPill® capsule (RT-114) (N=10) or subcutaneously (N=6), which is estimated to be approximately equivalent to a 60mg dose in humans.
- Endpoints included measures of safety, tolerability, and reliability of the RaniPill® capsule, as well as pharmacokinetics (serum drug concentration determined by ELISA) and pharmacodynamics (body weight and food intake).
Data Highlights
- The RaniPill® capsule was well tolerated with no changes in drug-related safety profile compared to subcutaneous delivery and was excreted without sequelae in all canines.
- RT-114 achieved a
90% delivery success rate (9 out of 10 canines). - RT-114 yielded higher Cmax, earlier Tmax, and a relative bioavailability of
111% compared to SC PG-102. - Average peak weight loss was the same in both groups with greater variability with SC dosing (
6.7% ±0.5% for RT-114 and6.7% ±2.2% for SC PG-102). - Rises in serum drug levels coincided with decreases in food consumption indicating strong PK-PD relationship.
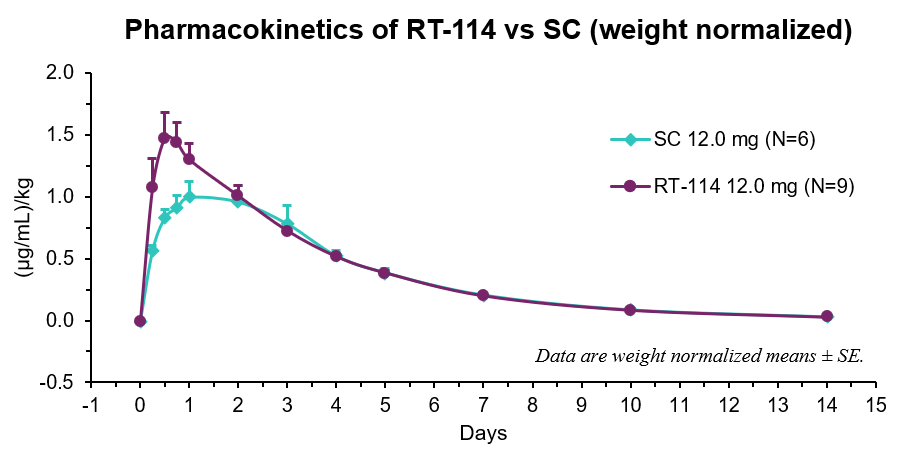
| Route | Cmax (µg/mL)/kg | Tmax (days) | AUClast (µg/mL*day)/kg |
| RT-114 | 1.51 ± 0.19 | 0.7 ± 0.1 | 5.48 ± 0.33 |
| SC | 1.06 ± 0.13 | 1.3 ± 0.3 | 4.92 ± 0.52 |
Near-Term Milestone Expectations:
- Initiation of Phase 1 clinical trial of RT-114 for the treatment of obesity expected in mid-2025.
About Rani Therapeutics
Rani Therapeutics is a clinical-stage biotherapeutics company focused on advancing technologies to enable the development of orally administered biologics and drugs. Rani has developed the RaniPill® capsule, which is a novel, proprietary and patented platform technology, intended to replace subcutaneous injection or intravenous infusion of biologics and drugs with oral dosing. Rani has successfully conducted several preclinical and clinical studies to evaluate safety, tolerability and bioavailability using RaniPill® capsule technology. For more information, visit ranitherapeutics.com.
About RT-114 Collaboration
RT-114 is the subject of a Collaboration Agreement between Rani and ProGen entered into in June 2024. Under the Collaboration Agreement, Rani and ProGen will collaborate to manufacture, develop, seek regulatory approvals for and, if approved, commercialize RT-114 in the field of weight management (including without limitation obesity, weight reduction and weight maintenance) in humans. Under the Collaboration Agreement, development costs, as well as operating profits and losses from the commercialization of RT-114, will be equally shared by Rani and ProGen. The parties share responsibility for the development of RT-114 worldwide, with Rani leading such development for preclinical activities through Phase 1 clinical trials. After initiation of the first Phase 2 clinical trial, Rani will lead development and commercialization of RT-114 in the United States, Canada, Europe (including the United Kingdom) and Australia, and ProGen will lead development and commercialization in all other countries.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, the expected initiation of a Phase 1 trial of RT-114 in mid-2025, the potential of the RaniPill® platform to enable oral delivery of multiple obesity treatments and validation of such potential through preclinical data, the potential for RT-114 to be a first-in-class orally administered GLP-1/GLP-2 dual agonist to treat obesity, the targeting of RT-114 to have a convenient oral dosing regimen for the treatment of obesity, the potential of PG-102’s GLP-1/GLP-2 dual agonist construct to induce higher quality weight loss, the potential for RT-114 to improve body composition and nutritional health of patients, the convenience and attractiveness of an oral RT-114 treatment for patients, the potential that the characteristics of PG-102 could lead to faster onset of effect and achievement of clinically meaningful weight loss, the sufficiency of Rani’s cash reserves, and future financial performance. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believe,” “would,” “potential,” “expect,” “targeting,” “may,” “could,” “look forward” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Rani’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks and uncertainties associated with Rani’s business in general and the other risks described in Rani’s filings with the Securities and Exchange Commission, including Rani’s annual report on Form 10-K for the year ended December 31, 2023, and subsequent filings and reports by Rani. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Rani undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
Investor Contact:
investors@ranitherapeutics.com
Media Contact:
media@ranitherapeutics.com
Graphs accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/a05200b3-2a45-4fde-b650-a37e283a2843
https://www.globenewswire.com/NewsRoom/AttachmentNg/29308181-ece1-4852-9667-287d84f843c6








