Propanc Biopharma’s CSO Reflects on Unique Anti-Cancer Effects of PRP Discovered Over Past Decade
Five Scientific Publications Underscores Proenzymes as an Effective Tool to Treat and Prevent Metastatic Cancer from Solid Tumors with
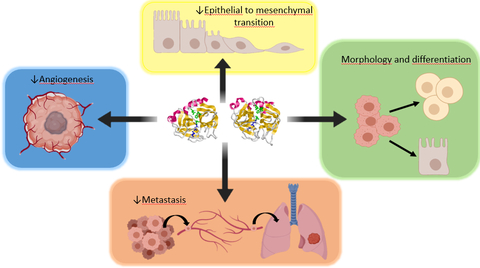
PRP represents a new advancement in the treatment of cancer by inducing cell differentiation, impairing angiogenesis, inhibiting cancer stem cell formation and blocking the EMT process. (Graphic: Business Wire)
Since 2013,
“Since our first peer reviewed scientific publication in 2013, which highlights how proenzymes suppresses the EMT program and promotes cell differentiation, I continue to be amazed at the compelling results achieved by our research team which demonstrates that we have a unique approach to treat and prevent metastatic cancer by targeting and eradicating cancer stem cells, whilst leaving healthy cells alone. This confirms what I observed clinically, when I first treated 46 late-stage cancer patients in a compassionate use study, which extended the survival of a number of patients, free from the serious side effects normally associated with standard treatment approaches. Since then, we have achieved proof of concept, in vivo (in a living organism), and completed translational development activities so that we can reproduce these scientific results in a randomized and controlled clinical study in advanced cancer patients.”
The joint scientific papers have been published in journals such as Cellular Oncology, Scientific Reports (an online Nature journal) and Expert Opinion on Biological Therapy. One paper, which explores in vivo pharmacokinetic (activity of drugs in an organism over time) studies and the anti-tumour efficacy of PRP against orthotopic (grafted) pancreatic and ovarian cancer tumours, as well as clinical observations from the compassionate use study conducted by
Further research is in progress with the Company’s joint research partners focusing on the effects of PRP on the tumor microenvironment. Results shows that PRP causes reversal of the malignant tumor phenotype (observable characteristics) towards a normal, or benign state, which was, “most unexpected, very exciting and powerfully conclusive,” according to Ms. Belen Toledo MSc, from the laboratory of Professor Macarena Perán PhD, University of
PRP is a mixture of two proenzymes, trypsinogen and chymotrypsinogen from bovine pancreas administered by intravenous injection. A synergistic ratio of 1:6 inhibits growth of most tumor cells. Examples include kidney, ovarian, breast, brain, prostate, colorectal, lung, liver, uterine and skin cancers.
About
The Company’s novel proenzyme therapy is based on the science that enzymes stimulate biological reactions in the body, especially enzymes secreted by the pancreas. These pancreatic enzymes could represent the body’s primary defense against cancer.
To view the Company’s “Mechanism of Action” video on its anti-cancer lead product candidate, PRP, please click on the following link: http://www.propanc.com/news-media/video
Forward-Looking Statements
All statements other than statements of historical facts contained in this press release are “forward-looking statements,” which may often, but not always, be identified by the use of such words as “may,” “might,” “will,” “will likely result,” “would,” “should,” “estimate,” “plan,” “project,” “forecast,” “intend,” “expect,” “anticipate,” “believe,” “seek,” “continue,” “target” or the negative of such terms or other similar expressions. These statements involve known and unknown risks, uncertainties and other factors, which may cause actual results, performance or achievements to differ materially from those expressed or implied by such statements. These factors include uncertainties as to the Company’s ability to continue as a going concern absent new debt or equity financings; the Company’s current reliance on substantial debt financing that it is unable to repay in cash; the Company’s ability to successfully remediate material weaknesses in its internal controls; the Company’s ability to reach research and development milestones as planned and within proposed budgets; the Company’s ability to control costs; the Company’s ability to obtain adequate new financing on reasonable terms; the Company’s ability to successfully initiate and complete clinical trials and its ability to successful develop PRP, its lead product candidate; the Company’s ability to obtain and maintain patent protection; the Company’s ability to recruit employees and directors with accounting and finance expertise; the Company’s dependence on third parties for services; the Company’s dependence on key executives; the impact of government regulations, including FDA regulations; the impact of any future litigation; the availability of capital; changes in economic conditions, competition; and other risks, including, but not limited to, those described in the Company’s periodic reports that are filed with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20220728005483/en/
Investor Relations and Media:
Mr.
irteam@propanc.com
+61-3-9882-0780
Source:







