Precigen Announces Positive Phase 1 Dose Escalation and Expansion Cohort Data for Investigational Off-the-Shelf PRGN-2012 AdenoVerse™ Immunotherapy in Patients with Recurrent Respiratory Papillomatosis
Rhea-AI Summary
Precigen (NASDAQ: PGEN) reported positive Phase 1 clinical trial results for its investigational PRGN-2012 AdenoVerse immunotherapy in patients with recurrent respiratory papillomatosis (RRP). Key findings include:
- 50% of patients achieved Complete Response, requiring no surgeries within 12 months post-treatment.
- Median surgeries decreased from 6.5 to 0.5 for treated patients.
- 32 patients are currently enrolled in Phase 2, with future regulatory discussions pending.
These results suggest a significant reduction in surgical intervention and a potential shift in treatment paradigms for RRP.
Positive
- 50% of patients achieved Complete Response at Dose Level 2, requiring no surgeries in the following 12 months.
- Median number of RRP surgeries declined from 6.5 pre-treatment to 0.5 post-treatment.
- 32 patients have been enrolled in the ongoing Phase 2 trial, indicating strong interest and progress.
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, PGEN declined 24.66%, reflecting a significant negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
– Repeated administrations of PRGN-2012 were well-tolerated with no dose-limiting toxicities and no treatment-related adverse events greater than Grade 2 –
– Clinical data show strong response in RRP patients with
– PRGN-2012 treatment at Dose Level 2 significantly reduced the need for surgeries in severe, aggressive RRP patients; Median number of RRP surgeries in 12-month period reduced from 6.5 pre-treatment to 0.5 post-treatment –
– Phase 2 study is enrolling patients with a total of 32 patients enrolled at Dose Level 2 to date –
– Company to outline regulatory strategy in RRP as
–
The company will host an R&D Day virtual event today at
"As a patient and an advocate on behalf of the RRP community, the potential for a therapeutic alternative to surgical intervention would be nothing short of life changing," said
"RRP is a rare disease with no cure. The current standard-of-care is repeated surgery to treat symptoms, which exposes patients to surgical risks, emotional distress and poses a significant economic burden to families and the healthcare system overall. We are thrilled to present these Phase 1 results today as PRGN-2012 has the potential to improve the lives of patients with severe, aggressive RRP through reduced surgeries," said
About RRP
RRP is a rare, difficult-to-treat and sometimes fatal neoplastic disease of the upper and lower respiratory tracts that is caused by infection with HPV 6 or HPV 11.1-4 RRP is classified based on age of onset as juvenile or adult. There is no approved therapeutic treatment for RRP and the current standard-of-care is repeated endoscopic debulking with ablation or excision of papillomatous lesions.3,4 Surgeries are not curative and recurrence of papilloma after surgical removal is very common and repeated procedures are required to debulk and monitor the disease, which exposes patients to anesthetic and surgical risks, and emotional distress. Patients with aggressive RRP can undergo hundreds of lifetime surgeries to control their disease.5 RRP morbidity and mortality results from the effects of papilloma mass on the vocal cords, trachea, and lungs, which may cause voice changes, stridor, airway occlusion, loss of lung volume, and/or post-obstructive pneumonia.6 Although rare, RRP has potential for malignant transformation in three to seven percent of adult patients.7
About PRGN-2012 AdenoVerse Immunotherapy
PRGN-2012 is an innovative therapeutic vaccine with optimized antigen design that uses
About the Phase 1 Clinical Trial
The Phase 1 clinical trial (clinical trial identifier: NCT04724980) evaluated safety and efficacy of PRGN-2012 as an immunotherapy following standard-of-care RRP surgery. Trial design included initiation of a 3+3 dose escalation cohort followed by a dose expansion cohort to enroll additional patients at the recommended Phase 2 dose (RP2D). Adult patients with severe, aggressive RRP, defined as greater than or equal to three surgeries in the prior 12 months, were enrolled to the clinical trial. A total of 15 patients were enrolled in the Phase 1 dose escalation and expansion cohorts at Dose Level 1: 1 x 1011 viral particles (vp)/dose (N=3) or Dose Level 2: 5 x 1011 vp/dose (N=12) with patients receiving four PRGN-2012 administrations (on days 1, 15, 43 and 85) via subcutaneous injection.
Patient Characteristics
Baseline patient characteristics of the 15 adult patients included a median age of 51 years (range: 30-73). Ten patients were male and 5 were female. Patients had an average of 6.2 surgeries (range: 3-10) in the last 12 months before enrolling in the trial. Patients were diagnosed with RRP for an average of 15 years prior to enrollment.
Safety Summary
Repeated administrations of PRGN-2012 were well-tolerated with no dose-limiting toxicities and no treatment-related adverse events (TRAEs) greater than Grade 2 (TABLE 1). All patients received four administrations of PRGN-2012 at the intended dose level. TRAEs were all mild and reduced in frequency over the treatment interval. The most common TRAE was injection site reaction, which occurred in all of the patients. Most other TRAEs occurring in more than one subject were similar to seasonal vaccines and the most common were fatigue, fever, and chills (TABLE 2). The lack of a significant neutralizing antibody response to gorilla adenovector over time with subsequent additional vaccinations highlights the ability to deliver repeated administrations of PRGN-2012, a differentiating feature of the AdenoVerse platform.
TABLE 1: Treatment-related Adverse Events | ||||||
Total Patients (N=15) | ||||||
Dose Level 1 1 x 1011 vp (N=3) | Dose Level 2 5 x 1011 vp (N=12) | All Subjects (N=15) | ||||
Subjects (N, %) | Events (N) | Subjects (N, %) | Events | Subjects (N, %) | Events (N) | |
Grade 1 | 3 (100 %) | 7 | 12 (100 %) | 105 | 15 (100 %) | 112 |
Grade 2 | 0 (0 %) | 0 | 2 (16.7 %) | 4 | 2 (13.3 %) | 4 |
Grades 3 - 5 | 0 (0 %) | 0 | 0 (0 %) | 0 | 0 (0 %) | 0 |
TABLE 2: Treatment-related Adverse Events by Grade | ||||
Total Patients (N=15) | ||||
Grade 1 | Grade 2 | |||
Subjects (N, %) | Events (N) | Subjects (N, %) | Events (N) | |
Chills | 10/15 ( | 14 | 0 (0 %) | 0 |
Diarrhea | 1/15 ( | 1 | 0 (0 %) | 0 |
Shortness of breath (Dyspnea) | 1/15 ( | 1 | 0 (0 %) | 0 |
Excessive sweating (Hyperhidrosis) | 2/15 ( | 2 | 0 (0 %) | 0 |
Fatigue | 9/15 ( | 20 | 2/15 ( | 2 |
Fever | 9/15 ( | 17 | 0 (0 %) | 0 |
Injection site reaction | 15/15 ( | 46 | 0 (0 %) | 0 |
Muscle aches (Myalgia) | 2/15 ( | 2 | 2/15 ( | 2 |
Nausea | 4/15 ( | 6 | 0 (0 %) | 0 |
Skin itching (Pruritus) | 1/15 ( | 1 | 0 (0 %) | 0 |
Vomiting | 2/15 ( | 2 | 0 (0 %) | 0 |
Clinical Efficacy Summary
Clinical data show PRGN-2012 treatment significantly reduced the need for surgeries for severe, aggressive RRP patients treated at Dose Level 2. At Dose Level 2,
Further, PRGN-2012 treatment showed significant improvement in anatomical Derkay scores, a tool used for research purposes to quantify RRP severity based on involvement of laryngeal structures, and voice quality, evaluated using the validated Vocal Handicap Index-10 (VHI-10), at 24-weeks post PRGN-2012 treatment completion compared to baseline.
Phase 1 data show that PRGN-2012 treatment resulted in an increase in HPV 6/11-specific T-cell response in the peripheral blood of RRP patients. Furthermore, the T-cells infiltrating papillomas from patients who had an objective response and a biopsy sample available showed an increase in HPV 6/11-specifc T-cells in papillomas after PRGN-2012 treatment.
TABLE 3: Clinical Efficacy Summary | ||
Total Patients (N=15) | ||
Dose Level 1 (N=3) | Dose Level 2 (N=12) | |
Complete Response (CR) No surgeries needed during 12-months post-treatment | ||
Overall Response Rate (ORR) ≥ | ||
Decrease in Rate of Surgery 12-months post-treatment compared to 12-months pre-treatment | ||
Case Studies
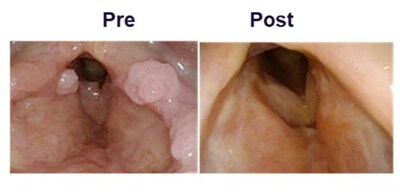
Case studies will be presented for three of the six complete responders. An example case study included subject #5, a 40 year old male who required eight surgeries in the 12-months prior to treatment to control papilloma growth. This patient has been in Complete Response after completing PRGN-2012 treatment, and has been surgery-free up to 18-months as of the cutoff date (FIGURE 1). Consistent with the disease response, the patient's Derkay score and VHI-10 index showed significant reduction at 24-weeks post treatment compared to baseline indicating improvement in disease severity and vocal function, respectively. The patient showed improvement in HPV-specific immune response in peripheral blood and papilloma post PRGN-2012 treatment.
Phase 2 Study
About AdenoVerse Immunotherapy
Trademarks
Cautionary Statement Regarding Forward-Looking Statements
Some of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T and AdenoVerse therapies. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the
References
1 Mounts, P et al. (1982). "Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx." Proc Natl Acad Sci U S A 79(17): 5425-5429.
2 Smith, E et al. (1993). "Human papillomavirus infection in papillomas and nondiseased respiratory sites of patients with recurrent respiratory papillomatosis using the polymerase chain reaction." Arch Otolaryngol Head Neck Surg 119(5): 554-557.
3 Derkay, CS et al. (2008). "Recurrent respiratory papillomatosis: a review." Laryngoscope 118(7): 1236-1247.
4 Derkay, CS et al. (2019). "Update on Recurrent Respiratory Papillomatosis." Otolaryngol Clin North Am 52(4): 669-679.
5 Allen, CT et al. (2019). "Safety and clinical activity of PD-L1 blockade in patients with aggressive recurrent respiratory papillomatosis." J. Immunotherapy Cancer 7, 119.
6 Seedat, RY (2020). "Juvenile-Onset Recurrent Respiratory Papillomatosis Diagnosis and Management - A Developing Country Review." Pediatric Health
7 Katsenos S, et al. (2011). "Recurrent respiratory papillomatosis: a rare chronic disease, difficult to treat, with potential to lung cancer transformation: apropos of two cases and a brief literature review." Case Rep Oncol. 2011 Mar 23;4(1):162-71.
Investor Contact:
Vice President, Investor Relations
Tel: +1 (301) 556-9850
investors@precigen.com
Media Contacts:
press@precigen.com
glenn.silver@finnpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-dose-escalation-and-expansion-cohort-data-for-investigational-off-the-shelf-prgn-2012-adenoverse-immunotherapy-in-patients-with-recurrent-respiratory-papillomatosis-301729690.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-positive-phase-1-dose-escalation-and-expansion-cohort-data-for-investigational-off-the-shelf-prgn-2012-adenoverse-immunotherapy-in-patients-with-recurrent-respiratory-papillomatosis-301729690.html
SOURCE