Penumbra and Asahi Intecc Partner With Goal of Introducing Indigo™ System Including Lightning™ Intelligent Aspiration to Japan
-
Companies aim to introduce Penumbra’s most advanced peripheral mechanical thrombectomy technology to
Japan upon regulatory approval - Lightning technology is designed for single session blood clot removal in peripheral arterial and venous systems, including for diseases such as acute limb ischemia, vein thrombosis and pulmonary embolism
- First and only computer-aided mechanical aspiration technology that can differentiate between clot and blood, which is designed to minimize blood loss
“By bringing together our newest innovations with Asahi’s leadership and expertise in the Japanese medical device market, we can provide physicians in
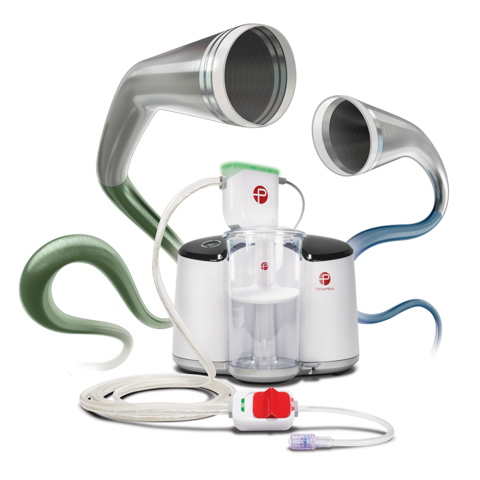
Penumbra’s Indigo Aspiration System can be used to remove emboli and thrombi from vessels of the peripheral arterial and venous systems, and for treatment of pulmonary embolism. A minimally-invasive device, Indigo enables the restoration of blood flow in such cases as acute limb ischemia and venous thrombus. Penumbra’s newest generation offering combines hypotube-based Indigo Aspiration Catheters with Lightning Intelligent Aspiration, a unique computer-aided clot detection technology that can differentiate between clot and blood, designed to reduce blood loss and the need for clot-dissolving drugs, which may lower the risk of bleeding complications.
“Our companies share a similar mission to help expand the available treatment options for serious conditions that impact an increasing number of people in Japan,” said
Penumbra’s neurovascular thrombectomy and neurovascular and peripheral embolization devices will continue to be distributed by its long-time trusted partner, Medico’s Hirata. This includes the latest RED™ reperfusion catheters, which are designed with optimized trackability and aspiration efficiency to help navigate the complex anatomy of the brain and deliver efficient aspiration for the removal of blood clots in a broad range of stroke patients.
About Penumbra
Important Safety Information
Additional information about Penumbra’s products can be located on Penumbra’s website at http://www.penumbrainc.com/healthcare-professionals. Prior to use, please refer to Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events and detailed instructions for use. Risk information can be found here.
Forward-Looking Statements
Except for historical information, certain statements in this press release are forward-looking in nature and are subject to risks, uncertainties and assumptions about us. Our business and operations are subject to a variety of risks and uncertainties and, consequently, actual results may differ materially from those projected by any forward-looking statements. Factors that could cause actual results to differ from those projected include, but are not limited to: the impact of the COVID-19 pandemic on our business, results of operations and financial condition; failure to sustain or grow profitability or generate positive cash flows; failure to effectively introduce and market new products; delays in product introductions; significant competition; inability to further penetrate our current customer base, expand our user base and increase the frequency of use of our products by our customers; inability to achieve or maintain satisfactory pricing and margins; manufacturing difficulties; permanent write-downs or write-offs of our inventory; product defects or failures; unfavorable outcomes in clinical trials; inability to maintain our culture as we grow; fluctuations in foreign currency exchange rates; potential adverse regulatory actions; and the potential impact of any acquisitions, mergers, dispositions, joint ventures or investments we may make. These risks and uncertainties, as well as others, are discussed in greater detail in our filings with the
Source:
View source version on businesswire.com: https://www.businesswire.com/news/home/20220922005290/en/
jheth@penumbrainc.com
510-995-9791
joni@merrymancommunications.com
323.532.0746
Source:







