Oculis Announces Positive OCS-05 Phase 2 ACUITY Trial in Acute Optic Neuritis, Met Primary Safety Endpoint and Key Secondary Efficacy Endpoints Opening Development Pathways as a Potential First-in-Class Neuroprotective Therapy
Rhea-AI Summary
Oculis announced positive topline results from its Phase 2 ACUITY trial for OCS-05, a potential first-in-class neuroprotective therapy for acute optic neuritis. The trial met its primary safety endpoint and achieved statistical significance on key efficacy endpoints.
The randomized, double-blind study evaluated 33 patients receiving OCS-05 (2mg/kg/day or 3mg/kg/day) administered intravenously for five days. Key results include:
- 43% improvement in GCIPL thickness at 3 months for OCS-05 (3mg/kg/day)
- 28-30% improvement in RNFL thickness at 3-6 months
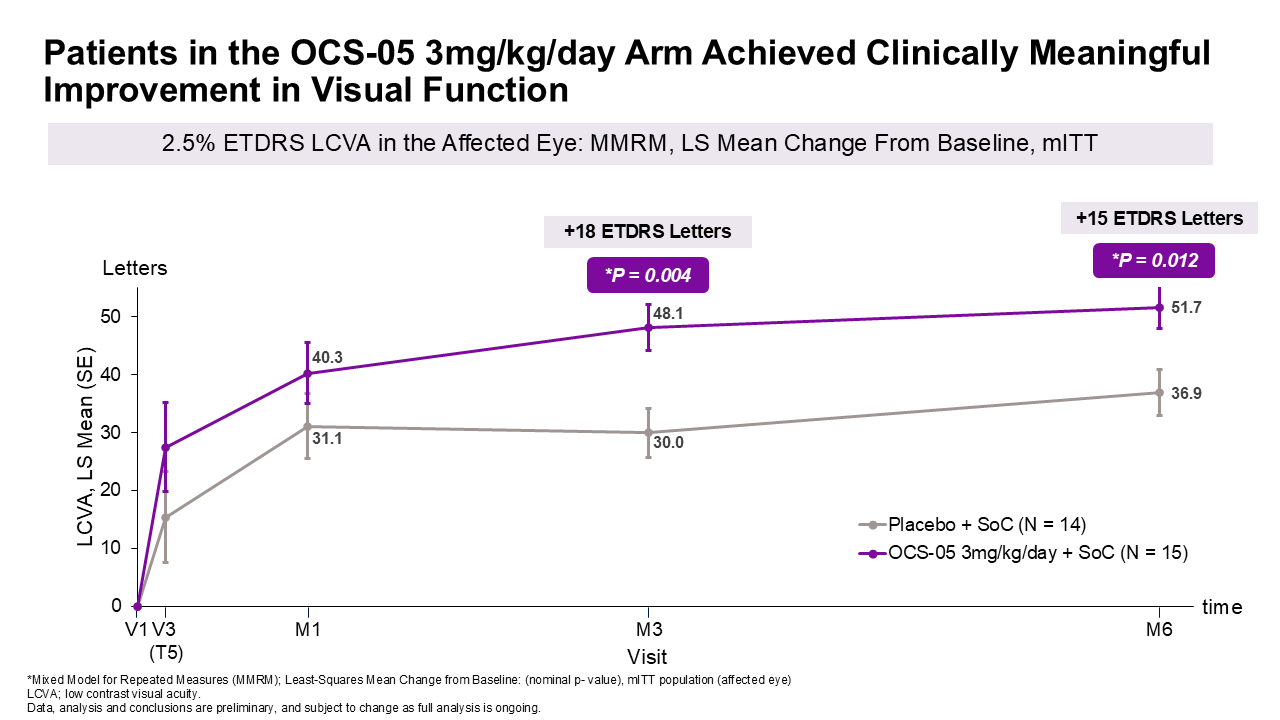
- Approximately 18 letters improvement in visual function at 3 months
The treatment showed a favorable safety profile with no serious adverse events or discontinuations. The FDA has granted orphan drug designation and cleared the IND application, enabling U.S. clinical development. The company reports approximately $105-110 million in cash and equivalents.
Positive
- Met primary safety endpoint with no serious adverse events
- Achieved 43% improvement in GCIPL thickness vs placebo at 3 months
- Showed 28-30% improvement in RNFL thickness vs placebo
- Demonstrated 18-letter improvement in visual function
- Received FDA orphan drug designation and IND clearance
- Strong cash position of $105-110 million
Negative
- Small trial size of only 33 treated patients
- Results need confirmation in larger clinical trials
News Market Reaction 1 Alert
On the day this news was published, OCS gained 0.51%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Oculis Announces Positive OCS-05 Phase 2 ACUITY Trial in Acute Optic Neuritis, Met Primary Safety Endpoint and Key Secondary Efficacy Endpoints Opening Development Pathways as a Potential First-in-Class Neuroprotective Therapy
- OCS-05 showed a favorable safety and tolerability profile compared to placebo
- Achieved statistically significant results on key secondary efficacy endpoints compared to placebo, including objective structural measures of retinal thickness and visual improvement
- IND clearance from FDA enables initiation of clinical development in the U.S as part of a global development program
- Planning next steps for OCS-05 as a potential first-in-class neuroprotective therapy for acute optic neuritis, while evaluating potential expansion into neurodegenerative diseases in ophthalmology and neurology
- Company to host investor and analyst webcast on January 6, 2025, at 8:00am Eastern Time
ZUG, Switzerland, January 6, 2025 – Oculis Holding AG (Nasdaq: OCS / ICX: OCS.IC) (“Oculis”), a global biopharmaceutical company purposefully driven to save sight and improve eye care, today announced positive topline results with OCS-05 in the Phase 2 ACUITY trial, which met the primary endpoint of safety and achieved statistical significance on several key efficacy-based secondary endpoints. The trial evaluated the safety, tolerability and efficacy of OCS-05, a neuroprotective candidate, in patients with acute optic neuritis.
The Phase 2 ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial was a randomized, double-blind, placebo-controlled, multi-center trial, designed to evaluate OCS-05 (2mg/kg/day or 3mg/kg/day) administered intravenously once-daily for five days in patients with acute optic neuritis also receiving steroid. The study randomized 36 patients with recent onset (visual loss symptoms) of unilateral acute optic neuritis with a demyelinating origin, of which 33 patients received treatment and were included in the pre-specified modified intent-to-treat (mITT) analysis.
Positive results from the ACUITY trial showed that OCS-05 achieved primary safety endpoint in addition to highlighting neuroprotective structural benefit and the ability to improve visual function in patients suffering from acute optic neuritis.
Primary Endpoint was Safety-Based:
The percentage of patients with a shift from normal (baseline) to abnormal in electrocardiogram (ECG) parameters after study drug administration until Visit 4 (Day 15) was measured to evaluate cardiac safety. The results showed no difference in the percentage of patients with abnormal ECG parameters between the two treatment arms.
- Two patients in the OCS-05 arms (2 and 3 mg/kg/day) and one patient in the placebo arm had a shift from normal to abnormal in any ECG measures between baseline and Visit 4 (Day 15), both equivalent to
12.5% . Events observed in the OCS-05 arms were mild and transient and qualified as not clinically significant by central review reading center.
Secondary Efficacy Endpoints Assessed Changes in Retinal Structure:
Optical Coherence Tomography (OCT) imaging was used to objectively measure the thickness of two different retinal segments in the affected eye to evaluate the potential neuroprotective effects of OCS-05 compared to placebo: 1) Ganglion Cell-Inner Plexiform Layer (GCIPL) and 2) Retinal Nerve Fiber Layer (RNFL). Results showed:
- A
43% improvement in GCIPL thickness mean change from baseline in favor of OCS-05 (3mg/kg/day) + steroid compared to placebo + steroid at month 3 which was maintained through month 6 with p-values* of 0.049 and 0.052 at 3 and 6 months, respectively. - A
28% improvement in RNFL thickness mean change from baseline in favor of OCS-05 (3mg/kg/day) + steroid compared to placebo + steroid at month 3 reaching30% improvement at month 6 with p-values* of 0.045 and 0.033 at 3 and 6 months, respectively.
Secondary Efficacy Endpoint Assessed Changes in Visual Function:
Changes in
- A favorable difference in LCVA mean change from baseline of approximately 18 letters at month 3 and approximately 15 letters at month 6 with OCS-05 (3 mg/kg/day) + steroid compared to placebo + steroid, with p-values^ of 0.004 and 0.012 at 3 and 6 months, respectively.
Treatment emergent adverse events (TEAEs):
- No drug-related serious adverse events (SAEs).
- No AEs leading to drug withdrawal or study discontinuation.
- Most frequently reported drug related AEs >
10% in the OCS-05 (2 or 3 mg/kg/day) + steroid treatment group were headache: 2 patients (10.5% ), and acne: 2 patients (10.5% ).
Riad Sherif, MD, Chief Executive Officer of Oculis, commented: “These positive safety and efficacy results from ACUITY represent a significant milestone in bringing the first potential neuroprotective treatment in ophthalmology to patients. The improvement in vision is especially encouraging, and the consistent improvement in retinal structure highlights the therapeutic potential of OCS-05 across multiple ophthalmic and neurological conditions. We are excited to further advance OCS-05’s development in acute optic neuritis, while actively exploring its potential in additional neuro-ophthalmic indications with the aim to deliver a first-in-class neuroprotective treatment option to patients.”
Mark Kupersmith, MD, Professor, Vice chair translational research, Chair NORDIC at Icahn School of Medicine at Mount Sinai Hospital, New York, added: “These groundbreaking results represent an important advancement for acute optic neuritis patients. Steroids have been used to treat the inflammation seen in acute optic neuritis, but don’t prevent persistent visual impairments or reduce structural loss. There remains a critical unmet need for neuroprotective therapies to preserve vision and the potential neuroprotective properties of OCS-05 observed in the ACUITY trial and its impact on visual function could offer significant hope for patients. These results, if replicated in larger clinical trials, could have profound implications, not only for this condition, but potentially for MS and other optic nerve disorders as well as glaucoma.”
Pablo Villoslada, M.D., Chair of the Department of Neurology at Hospital del Mar, Pompeu Fabra University in Barcelona, Spain, Oculis’ Scientific Advisory Board Member, said: “I am excited to see that the ACUITY results are consistent with the robust effects observed in animal models of neuroinflammation and neurodegeneration in the prevention of retinal ganglion cell damage and that OCS-05 has shown promising results in the improvement of vision. I look forward to the additional studies that can now be initiated for acute optic neuritis while further exploring the full potential of this promising neuroprotective candidate in both ophthalmology and neurology indications.”
OCS-05 has received orphan drug designation from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for acute optic neuritis, a rare condition characterized by acute inflammation and demyelination of the optic nerve, often affecting young adults, in which retinal thinning is directly associated with vision loss and permanent visual impairment. There are currently no approved therapies specifically indicated for acute optic neuritis and despite steroids being used to treat inflammation and improve recovery, steroids are unable to provide neuroprotection to prevent vision loss.
In addition, the Investigational New Drug (IND) application for OCS-05 has also been cleared by the U.S. Food and Drug Administration (FDA), enabling the initiation of clinical development in the United States to support the global potential of OCS-05. The encouraging ACUITY trial results, Oculis’ positive development momentum and a solid balance sheet, including approximately
Analyst and investor call
The Oculis management team will host an analyst and investor call today at 8:00am U.S. Eastern Time, to review the trial results. Interested parties may participate in the call via the following webcast here.
A replay of the webcast and accompanying slides will be available for 90 days following the event through the “Events and Presentations” page of the “Investors and Media” section of the company’s website.
-ENDS-
About OCS-05
OCS-05 is a novel peptidomimetic small molecule candidate with the potential to become a first-in-class neuroprotective therapy for acute optic neuritis and other neuro-ophthalmic diseases. In animal models of neuroinflammation and neurodegeneration, OCS-05 has shown positive results in prevention of retinal ganglion cell damage and was associated with improvements in mobility (clinical function disability). OCS-05 is currently in Phase 2 development for the treatment of acute optic neuritis and has received orphan drug designation from both the FDA and the EMA. In addition to acute optic neuritis, a neuroprotective treatment could potentially have wide applicability in neuro-ophthalmic indications such as glaucoma, diabetic retinopathy and other vision-threatening diseases.
OCS-05 is an investigational drug and has not received regulatory approval for commercial use in any country.
About Acute Optic Neuritis
Acute optic neuritis is a rare condition characterized by an acute inflammation of the optic nerve that can lead to permanent visual impairment. It affects up to 8 in 100,000 people worldwide and often represents the first sign of multiple sclerosis. It mainly occurs in adults between the age of 20 and 40 years and is more frequent in women (2:1). The acute inflammatory process of acute optic neuritis leads to the loss of myelin covering the optic nerve and the axons. At the onset, patients often suffer from ocular pain that increases with eye movement and vision loss. Once the inflammation recedes, remyelination often occurs but it is incomplete. Without the myelin sheath protecting the axon, neurons located in demyelinated segments become fragile and prone to death. Unfortunately, damaged axons cannot regrow, leading to permanent visual impairment. To date there is no specific therapy approved for acute optic neuritis and unmet needs remain for therapies that can prevent vision loss after an acute episode of optic neuritis.
About ACUITY Trial
The Phase 2 ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial was a randomized, double-blind, placebo-controlled, multi-center trial in France, designed to evaluate a once-daily intravenous infusion of OCS-05 over five days compared with placebo, in patients with acute optic neuritis receiving steroids. In addition to safety, other secondary efficacy endpoints were measured to evaluate the potential of OCS-05 on neuroprotection and visual function improvement in acute optic neuritis patients. The study randomized 36 eligible patients aged between 18 to 60, with recent onset (visual loss symptoms) of unilateral acute optic neuritis with a demyelinating origin, of which 33 patients received OCS-05 2mg/kg/day, 3m/kg/day, or placebo for five days in addition to steroid and were included in the pre-specified mITT analysis.
The assessment of the primary safety endpoint was carried out through the percentage of patients with shift from normal (at baseline) to abnormal in any ECG parameter at any time from Day 1 (after study drug administration) to Day 15.
Secondary efficacy endpoints included:
- Change in ganglion cell and inner plexiform layer (GCIPL) thickness in the affected eye measured with OCT at central reading center at Day 5, Month 1, Month 3 and Month 6 compared to baseline of the affected eye
- The retinal ganglion cell layer, or retinal ganglion cell and inner plexiform layers combined (GCIPL), the 2 layers are difficult to discriminate by OCT, is not affected by disk swelling during the inflammation phase of acute optic neuritis.1
- Change in retinal nerve fiber layer (RNFL) thickness in the affected eye as measured with OCT at central reading center at Day 5, Month 1, Month 3 and Month 6 compared to baseline of the affected eye.
- The RNFL comprises of unmyelinated axons originating from the retinal ganglion cell bodies. During acute optic neuritis onset, RNFL thickness first increases due to optic nerve swelling during inflammation, but post-inflammation reduction in thickness indicates axonal loss. Because RNFL is not myelinated, its thinning is direct evidence of axonal loss, as opposed to a potential demyelination and loss of oligodendrocytes.2,3,4
- Change in the
2.5% ETDRS low contrast letter acuity (LCVA) measured at Day 15, Month 1, Month 3, and Month 6 in the affected eye compared to baseline.- LCVA better captures the persistent decrement in low contrast after acute optic neuritis and overcomes the ceiling effect observed with high contrast visual acuity (HCVA) / best corrected visual acuity (BCVA) measurement.5
More information about the study is available on ClinicalTrials.gov here.
About Oculis
Oculis is a global biopharmaceutical company (Nasdaq: OCS / ICX: OCS.IC) purposefully driven to save sight and improve eye care. Oculis’ highly differentiated pipeline comprises multiple innovative product candidates in development. It includes OCS-01, a topical eye drop candidate for diabetic macular edema (DME) and for the treatment of inflammation and pain following cataract surgery; OCS-02 (licaminlimab), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and for non-infectious anterior uveitis; and OCS-05, a candidate designed to be neuroprotective for acute optic neuritis. Headquartered in Switzerland and with operations in the U.S. and Iceland, Oculis’ goal is to improve the health and quality of life of patients worldwide. The company is led by an experienced management team with a successful track record and is supported by leading international healthcare investors.
For more information, please visit: www.oculis.com
Oculis Contacts
Ms. Sylvia Cheung, CFO
sylvia.cheung@oculis.com
Investor Relations
LifeSci Advisors
Corey Davis, Ph.D.
cdavis@lifesciadvisors.com
1-212-915-2577
Media Relations
ICR Healthcare
Amber Fennell / David Daley / Sean Leous
oculis@icrhealthcare.com
Cautionary Statement Regarding Forward Looking Statements
This financial information in this release reflects the Company’s preliminary estimate, based on currently available information. Financial closing procedures for the quarter are not yet completed and final results may therefore vary from this estimate. This preliminary estimate has not been audited by our independent registered public accounting firm.
This press release contains forward-looking statements and information. For example, statements regarding the potential effects of OCS-05, including patient impact and market opportunity; the potential of OCS-05 to be a neuroprotective therapy or treatment for acute optic neuritis and other neuro-ophthalmic diseases; the potential of OCS-05 to promote neuronal survival and repair; the potential neuroprotective effects of OCS-05 on preserving retinal thickness in acute optic neuritis patients; the potential of OCS-05 to prevent vision loss; the Company’s estimated cash, cash equivalents and short-term investments; and Oculis’ research and development programs, regulatory and business strategy, future development plans, and management, are forward-looking. Certain clinical trial results presented in this press release are topline and preliminary and subject to change, as analysis is ongoing. These topline results may not be reproduced in subsequent patients and clinical trials. All forward-looking statements are based on estimates and assumptions that, while considered reasonable by Oculis and its management, are inherently uncertain and are inherently subject to risks, variability, and contingencies, many of which are beyond Oculis’ control. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, assurance, prediction or definitive statement of a fact or probability. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. All forward-looking statements are subject to risks, uncertainties and other factors that may cause actual results to differ materially from those that we expected and/or those expressed or implied by such forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of Oculis, including those set forth in the Risk Factors section of Oculis’ annual report on Form 20-F and any other documents filed with the U.S. Securities and Exchange Commission (the “SEC”). Copies of these documents are available on the SEC’s website, www.sec.gov. Oculis undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
1 Toosy, A. T., and al. (2014). Optic neuritis. The Lancet Neurology, 13(1), 83-99.
2 Trip, S. A., and al. (2005). Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Annals of neurology, 58(3), 383-391.
3 Costello, F., and al. (2006). Quantifying axonal loss after optic neuritis with optical coherence tomography. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 59(6), 963-969.
4 Henderson, A. P., and al. (2010). A serial study of retinal changes following optic neuritis with sample size estimates for acute neuroprotection trials. Brain, 133 (9), 2592-2602.
5 Petzold, A., and al. (2020). Case for a new corticosteroid treatment trial in optic neuritis: review of updated evidence. Journal of Neurology, Neurosurgery & Psychiatry, 91(1), 9-14.
* Mixed Model for Repeated Measures (MMRM) Least-Squares Mean Change from Baseline: (nominal directional p- value), mITT population (study eye)
^ Mixed Model for Repeated Measures (MMRM) Least-Squares Mean Change from Baseline: (nominal p- value), mITT population (study eye)






