NeuroBo Pharmaceuticals' DA-1726 Demonstrated Superiority in Weight Loss, Retention of Lean Body Mass, and Lipid-Lowering Effects Compared to Survodutide, in Pre-Clinical Models
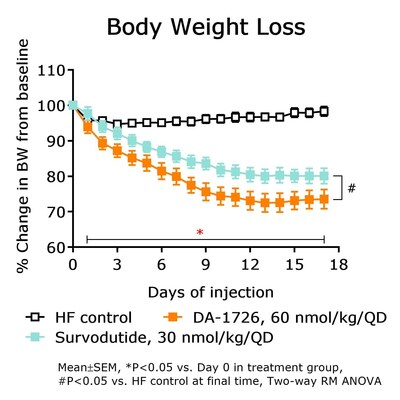
NeuroBo Pharmaceuticals revealed pre-clinical data for DA-1726, a new dual oxyntomodulin analog agonist, showing superior weight loss, lean body mass retention, and lipid-lowering effects compared to survodutide. In obese mouse models, DA-1726 significantly outperformed survodutide in reducing cholesterol (T-CHO: -67.7% vs. -49.6%) and triglycerides (TG: -49.5% vs. -41.2%). DA-1726 also demonstrated better glucose-lowering effects (-54.7% vs. -30.4% of survodutide). The drug's efficacy was tested at the ADA 84th Scientific Sessions in Orlando, June 21-24, 2024. The Phase 1 trial is ongoing, with initial dosing in Q3 and top-line data expected in Q1 2025.
- DA-1726 showed superior weight loss and lean body mass retention compared to survodutide.
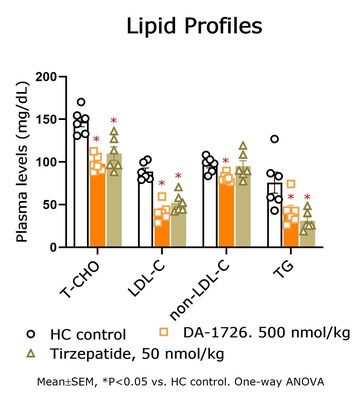
- Significant cholesterol and triglyceride reduction were noted with DA-1726 (-67.7%, -49.5%) vs. survodutide (-49.6%, -41.2%).
- Superior glucose-lowering effects with DA-1726 (-54.7%) compared to survodutide (-30.4%).
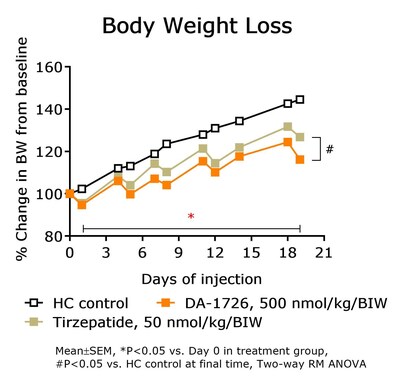
- DA-1726 outperformed tirzepatide in suppressing cholesterol and LDL levels in hypercholesterolemia rat models.
- Results are based on pre-clinical models; clinical effectiveness in humans remains unproven.
- Potential risks associated with transitioning from pre-clinical to clinical stages could affect stock performance.
Insights
DA-1726, a dual oxyntomodulin analog agonist, shows promising results in pre-clinical models that could potentially offer significant advantages over existing obesity treatments. The pre-clinical data indicates that DA-1726 demonstrated superior weight loss, lipid-lowering effects and glucose-lowering capabilities compared to survodutide and tirzepatide, which are drugs in the same class. Such results suggest the potential for DA-1726 to offer a more effective solution for obesity and related metabolic disorders.
A critical factor appears to be DA-1726's GLP-1 and glucagon receptor activity ratio. The glucagon action of DA-1726 may contribute to enhanced energy expenditure (EE) and the superior lipid-lowering effects observed. This characteristic could make DA-1726 a more effective option in terms of both weight management and lipid profile improvement.
When interpreting these findings, it's important to consider that they are based on pre-clinical animal models. While these results are promising, further validation in human clinical trials is necessary. The upcoming Phase 1 trial data will serve as a important next step in understanding the potential human efficacy and safety profile of DA-1726. Investors should look out for these results expected in the third quarter of this year, with additional data anticipated in early 2025.
In summary, DA-1726 could represent a significant advancement in obesity treatment if the favorable pre-clinical findings translate into human trials. However, caution is warranted until clinical data confirms these initial results.
The biotech sector is highly competitive, especially in the field of obesity treatments. A novel drug like DA-1726 that shows superior weight loss and lipid-lowering effects in pre-clinical models can potentially capture a significant market share if it proves effective in human trials. The obesity treatment market is expected to grow considerably over the next decade, driven by increasing obesity rates and the demand for more effective treatments.
Given the upcoming milestones—including the expected dosing of the first patient in the multiple ascending dose (MAD) Part 2 and the release of top-line data from the single ascending dose (SAD) Part 1—NeuroBo Pharmaceuticals is at a pivotal point. Positive results could trigger substantial interest from investors and potential partnerships or acquisitions by larger pharmaceutical companies looking to expand their obesity treatment portfolios.
However, retail investors should be aware of the inherent risks associated with investing in clinical-stage biotech companies. The transition from pre-clinical success to clinical efficacy and safety is fraught with challenges. Initial enthusiasm should be tempered with an understanding of the timeframes and hurdles involved in drug development.
In conclusion, the pre-clinical success of DA-1726 positions NeuroBo Pharmaceuticals as a company to watch, but prudent investment strategies should account for the high-risk, high-reward nature of biotech ventures.
NeuroBo Pharmaceuticals' announcement of promising pre-clinical data for DA-1726 could have a significant impact on its stock valuation. The positive pre-clinical results indicating superior efficacy in weight loss, lipid-lowering and glucose-lowering effects compared to existing drugs like survodutide and tirzepatide provide a strong potential value proposition.
In the short term, this could lead to increased investor interest and a potential stock price appreciation, particularly as we approach the release of clinical trial data later this year. Investors should monitor the upcoming milestones closely, as the Phase 1 trial outcomes will be important in determining the drug's future development and market potential.
Long-term implications depend significantly on the successful transition from pre-clinical to clinical stages. Should DA-1726 demonstrate similar efficacy in human trials, it could position NeuroBo Pharmaceuticals as a leader in the obesity treatment market, potentially leading to substantial revenue growth and market expansion opportunities.
However, the financial outlook should be balanced with an understanding of the typical risks associated with biotech stocks, such as regulatory hurdles and potential delays in clinical trials. Investors should also consider NeuroBo's current financial health and funding situation to ensure the company can adequately support the ongoing and future phases of clinical development.
Overall, NeuroBo Pharmaceuticals presents a potentially lucrative but high-risk investment opportunity, contingent on the successful clinical advancement of DA-1726.
DA-1726 Also Exhibited Superior Glucose Lowering Compared to Survodutide
Lipid-Lowering Effect of DA-1726 Shown to be Superior Compared to Tirzepatide
Data Presented at the ADA 84th Scientific Sessions
"The data being presented at the ADA further differentiates DA-1726 from obesity drugs in the same class, potentially due to its GLP-1 and glucagon receptor activity ratio," stated Hyung Heon Kim, President and Chief Executive Officer of NeuroBo. "DA-1726, in obese mouse models, significantly lowered cholesterol levels and induced superior weight loss, compared to survodutide, a drug with the same mechanism of action, while also exhibiting superior glucose lowering. Most notably, DA-1726 demonstrated superior weight loss and retention of relative lean body mass preservation compared to survodutide. Further, as previously disclosed pre-clinical studies showed, DA-1726 resulted in similar weight reduction while consuming more food compared to tirzepatide and this additional data, being presented at the ADA, in a hypercholesterolemia rat model, confirmed that DA-1726 is more effective than tirzepatide in suppressing cholesterol levels, due to its glucagon action, alongside its GLP-1 effect, while also inhibiting weight gain. We believe these distinguishing factors can potentially position DA-1726 as a best-in-class obesity drug with superior efficacy and better tolerability profile. The Phase 1 trial of DA-1726 is progressing well, and we expect to both dose the first patient in the multiple ascending dose (MAD) Part 2 and read-out top-line data from the single ascending dose (SAD) Part 1 in the third quarter of this year, with top-line data from the MAD Part 2 expected in the first quarter of 2025."
- Abstract Title: DA-1726, a GLP1R/GCGR Dual Agonist, A Promising Approach in Obesity Treatment and Lipid Management
- Presenter Name: Tae-Hyoung Kim, Lead research scientist, Dong-A ST Research Center
- Authors: Tae-Hyoung Kim, Il-Hun Jung, Su Jin Lee, Hyung Heon Kim, Mi-Kyung Kim, Yuna Chae
- Abstract Number: 2024-LB-5728
- Poster Presentation Number: 2058-LB
- Session: 23-A Obesity-Animal
- Session Date: Saturday, June 22, 2024
- Session Time: 12:30 pm – 1:30 pm ET
DA-1726 is currently in a Phase 1 clinical trial. This study focuses on the pharmacological effects of this novel oxyntomodulin analogue, which has been effective at improving lipid profiles and reducing weight in rodent models. In an obese mouse model, DA-1726 showed superior weight loss compared to survodutide (-
In a prior study, DA-1726 showed a difference in improving lipid levels, despite similar weight loss to tirzepatide. Therefore, the direct lipid-regulating effect of DA-1726 was assessed in a hypercholesterolemia rat model compared with tirzepatide. As a result, DA-1726 was more effective than tirzepatide in suppressing the elevation of T-CHO (-
In summary, it was confirmed that DA-1726 has differentiating characteristics in the competition of obesity treatment drugs with similar or identical mechanisms in its effect on improving cholesterol metabolism through glucagon action.
After the presentations, the poster will be accessible within the "Posters" section of NeuroBo's website at: https://www.neurobopharma.com/posters.
About DA-1726
DA-1726 is a novel oxyntomodulin (OXM) analogue functioning as a GLP1R/GCGR dual agonist for the treatment of obesity and Metabolic Dysfunction-Associated Steatohepatitis (MASH) that is to be administered once weekly subcutaneously. DA-1726 acts as a dual agonist of GLP-1 receptors (GLP1R) and glucagon receptors (GCGR), leading to weight loss through reduced appetite and increased energy expenditure. DA-1726 has a well understood mechanism and, in pre-clinical mice models, resulted in improved weight loss compared to semaglutide and cotadutide (another OXM analogue). Additionally, in pre-clinical mouse models, DA-1726 elicited similar weight reduction, while consuming more food, compared to tirzepatide and survodutide, while also preserving lean body mass and demonstrating lipid-lowering effects compared to survodutide.
About NeuroBo Pharmaceuticals
NeuroBo Pharmaceuticals, Inc. is a clinical-stage biotechnology company focused on transforming cardiometabolic diseases. The company is currently developing DA-1241 for the treatment of Metabolic Dysfunction-Associated Steatohepatitis (MASH) and is developing DA-1726 for the treatment of obesity. DA-1241 is a novel G-protein-coupled receptor 119 (GPR119) agonist that promotes the release of key gut peptides GLP-1, GIP, and PYY. In pre-clinical studies, DA-1241 demonstrated a positive effect on liver inflammation, lipid metabolism, weight loss, and glucose metabolism, reducing hepatic steatosis, hepatic inflammation, and liver fibrosis, while also improving glucose control. DA-1726 is a novel oxyntomodulin (OXM) analogue that functions as a glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR) dual agonist. OXM is a naturally-occurring gut hormone that activates GLP1R and GCGR, thereby decreasing food intake while increasing energy expenditure, thus potentially resulting in superior body weight loss compared to selective GLP1R agonists.
For more information, please visit www.neurobopharma.com.
Forward Looking Statements
Certain statements in this press release may be considered forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "believes", "expects", "anticipates", "may", "will", "should", "seeks", "approximately", "intends", "projects", "plans", "estimates" or the negative of these words or other comparable terminology (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this press release, including, without limitation, those risks associated with NeuroBo's ability to execute on its commercial strategy; the timeline for regulatory submissions; the ability to obtain regulatory approval through the development steps of NeuroBo's current and future product candidates, the ability to realize the benefits of the license agreement with Dong-A ST Co. Ltd., including the impact on future financial and operating results of NeuroBo; the cooperation of NeuroBo's contract manufacturers, clinical study partners and others involved in the development of NeuroBo's current and future product candidates; potential negative interactions between NeuroBo's product candidates and any other products with which they are combined for treatment; NeuroBo's ability to initiate and complete clinical trials on a timely basis; NeuroBo's ability to recruit subjects for its clinical trials; whether NeuroBo receives results from NeuroBo's clinical trials that are consistent with the results of pre-clinical and previous clinical trials; impact of costs related to the license agreement, known and unknown, including costs of any litigation or regulatory actions relating to the license agreement; the effects of changes in applicable laws or regulations; the effects of changes to NeuroBo's stock price on the terms of the license agreement and any future fundraising; and other risks and uncertainties described in NeuroBo's filings with the Securities Exchange Commission, including NeuroBo's most recent Annual Report on Form 10-K. Forward-looking statements speak only as of the date when made. NeuroBo does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Contacts:
NeuroBo Pharmaceuticals
Marshall H. Woodworth
Chief Financial Officer
+1-857-299-1033
marshall.woodworth@neurobopharma.com
Rx Communications Group
Michael Miller
+1-917-633-6086
mmiller@rxir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1726-demonstrated-superiority-in-weight-loss-retention-of-lean-body-mass-and-lipid-lowering-effects-compared-to-survodutide-in-pre-clinical-models-302179186.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-da-1726-demonstrated-superiority-in-weight-loss-retention-of-lean-body-mass-and-lipid-lowering-effects-compared-to-survodutide-in-pre-clinical-models-302179186.html
SOURCE NeuroBo Pharmaceuticals, Inc.







![DA-1726 [W0], DA-1726 [W2], Survodutide [W0], Survodutide [W2] DA-1726 [W0], DA-1726 [W2], Survodutide [W0], Survodutide [W2]](https://mma.prnewswire.com/media/2445098/pie_chart.jpg)