Molecular Templates, Inc. Provides Interim Update
- None.
- None.
Insights
The recent update from Molecular Templates regarding their engineered toxin bodies (ETBs) targeting PD-L1 and CTLA-4 in head and neck cancer is a significant development in the field of oncology. PD-L1 inhibitors are already a cornerstone in cancer immunotherapy and the emergence of ETBs may offer a differentiated mechanism of action that could complement or enhance current treatments. The reported monotherapy activity in heavily pre-treated patients suggests potential for these therapies in refractory cases where standard treatments have failed. However, it is crucial to consider the complexity of cancer biology and the challenges in predicting response rates across a larger population. Long-term studies and larger clinical trials will be essential to establish the safety profile, efficacy and potential resistance mechanisms associated with these novel therapies.
From a research perspective, the pharmacodynamic effects of MT-8421, particularly in its ability to target CTLA-4, could indicate a promising avenue for combination therapies. CTLA-4 is an immune checkpoint that, when inhibited, can enhance T-cell activation and proliferation, potentially leading to a more robust anti-tumor response. The ongoing phase I study will be pivotal in determining the optimal dosing and therapeutic window. It's important to note that while early phase studies can be promising, the transition to later-phase trials is fraught with increased scrutiny on safety and efficacy. Investors should monitor the progression of these trials closely, as positive data could lead to significant valuation inflections for MTEM.
For stakeholders in the biopharmaceutical sector, Molecular Templates' update is a noteworthy event. Early positive indicators from clinical trials can have a substantial impact on a company's stock performance, particularly for clinical-stage companies like MTEM. The specificity of ETBs in targeting cancer cells while sparing healthy ones could position Molecular Templates favorably in a competitive oncology market. However, investors should weigh the inherent risks of drug development, including the potential for adverse events, regulatory hurdles and the need for substantial capital to fund ongoing research and trials. The long-term financial implications will hinge on the successful completion of clinical trials, regulatory approvals and the ability to secure partnerships or funding for further development.
Monotherapy activity with PD-L1-targeting MT-6402 in Head and Neck Cancer; Unique pharmacodynamic effects with CTLA-4 targeting MT-8421 in on-going phase I study
AUSTIN, Texas, March 04, 2024 (GLOBE NEWSWIRE) -- Molecular Templates, Inc. (Nasdaq: MTEM, “Molecular Templates,” or “MTEM”), a clinical-stage biopharmaceutical company focused on the discovery and development of proprietary targeted biologic therapeutics, engineered toxin bodies (“ETBs”), to create novel therapies with potent differentiated mechanisms of action for cancer, today provided an update on its programs.
Eric Poma, PhD., Chief Executive and Chief Scientific Officer of MTEM, stated, “ETBs represent a new approach to oncology drug development that continue to show unique biology and monotherapy activity in heavily pre-treated patients. We are particularly excited about the monotherapy activity observed with our MT-6402 program in patients with head and neck cancer.”
Company Highlights
- Continued single agent activity observed with MT-6402 particularly in heavily pre-treated patients with head and neck cancer who had progressed on multiple prior therapies including checkpoint antibodies.
- The first dose of 32 mcg/kg has been cleared with no grade 3 or grade 4 drug-related toxicities in the phase I study for MT-8421 targeting CTLA-4-expressing regulatory T-cells (“Tregs”) in the tumor microenvironment. Unique pharmacodynamic effects demonstrating potent Treg clearance and IL-2 increases were observed at the first dose level. Enrollment in the second dose cohort (48 mcg/kg) is on-going.
- MTEM intends to initiate a study of MT-0169 in CD38+ acute leukemias in collaboration with MD Anderson Cancer Center.
- Preclinical activities related to Bristol Myers Squibb collaboration are on-going.
MT-6402 (PD-L1 ETB)
The Part A dose escalation of the phase I study for MT-6402 has been completed with no Grade 4 or Grade 5 drug-related adverse events observed to date.
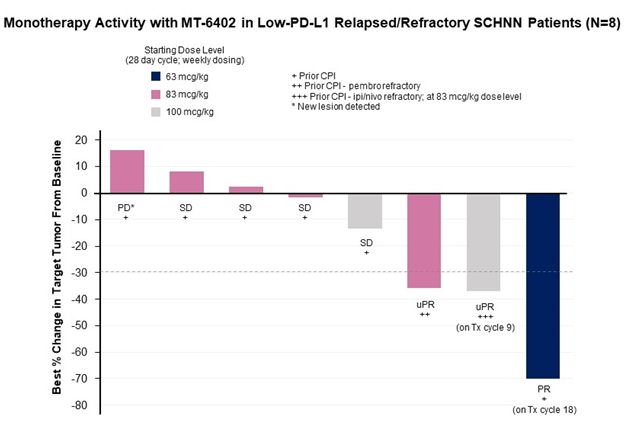
In the Part A dose escalation, 10 patients with head and neck squamous cell cancer (HNSCC) were treated at doses of 63, 83, or 100 mcg/kg. Two of these patients were not evaluable for the cycle 1 dose-limiting toxicity (“DLT”) period or for efficacy because of early progression and came off study after receiving only one or two doses of MT-6402, respectively. Of the remaining eight head and neck cancer patients, the best responses observed were as follows: three had a partial response (“PR”) (two unconfirmed) and a fourth patient had evidence of tumor regression. All four patients had progressed on their previous therapies after multiple lines of treatment including checkpoint antibodies. Additional details on each of these participant’s clinical profile and response to the investigational treatment are provided below.
- 1 patient with a PD-L1 TPS of
2% who had progressed after chemotherapy, radiation therapy, and pembrolizumab had a confirmed PR with70% tumor reduction and remains on study in cycle 18 (1 cycle = 4 weeks). - 1 patient with a PD-L1 CPS of
10% who had progressed after three previous lines of therapy, including progression on Ipi/Nivo within 4 months, showed deepening tumor reduction over time of3% ,9% , and15% at the end of cycles 2, 4, and 6, respectively. At the end of cycle 8, the patient had an unconfirmed PR with a37% reduction in tumor size. The patient remains on study in cycle 9. - 1 patient with a PD-L1 CPS of
5% who had progressed after six prior lines of therapy and was refractory to pembrolizumab received 2 doses of MT-6402 before coming off treatment due to the treating physician’s concerns around an asymptomatic grade 1 high sensitivity troponin elevation and hyponatremia related to excessive alcohol intake. The patient discontinued treatment, but a subsequent CT scan assessed by an external radiology review showed that the patient had a36% tumor reduction (an unconfirmed PR). - 1 patient with a PD-L1 CPS of
10% with pre-existing cardiac risk factors of hypertension, hyperlipidemia, and hypercholesterolemia received three doses of MT-6402 before presenting with asymptomatic grade 1 high sensitivity troponin elevation and dosing was held. A CT scan showed a13% reduction in tumor size, but disease progression occurred during treatment interruption and patient discontinued at the end of cycle 6.
The three other HNSCC patients enrolled in the Part A dose escalation had stable disease of 6, 4, and 2 months, respectively, before disease progression or study discontinuation. One patient progressed at the end of cycle 2. Of these 8 patients, only one patient (the patient with stable disease through 6 cycles) had a PD-L1 tumor proportion score (“TPS”) greater than
“We are very excited to see objective responses in heavily pre-treated, checkpoint-experienced, head and neck cancer patients, a setting with high unmet medical need,” said Eric Poma. “Current checkpoint monotherapy in I/O-naïve head and neck cancer patients has a ~
“MT-6402 appears generally well-tolerated at the 63 and 83 mcg/kg doses with no Grade 4 or Grade 5 adverse events and no instances of CLS seen at any dose,” said Dr. Maurizio Voi, Chief Medical Officer of Molecular Templates. “The irAE profile of MT-6402, including the asymptomatic high sensitivity troponin elevations, appears to be consistent with that seen with other checkpoint therapies.” The Part B dose expansion portion of the phase I study in patients with high PD-L1 is ongoing.
MT-8421 (CTLA-4 ETB)
- MT-8421, along with MT-6402, represent our unique approach to immuno-oncology based on dismantling the TME through the elimination of immunosuppressive cells in the TME.
- MT-8421 is designed to potently destroy CTLA4+ Tregs via enzymatic ribosome destruction but does not have activity against low CTLA-4 expressing peripheral Tregs.
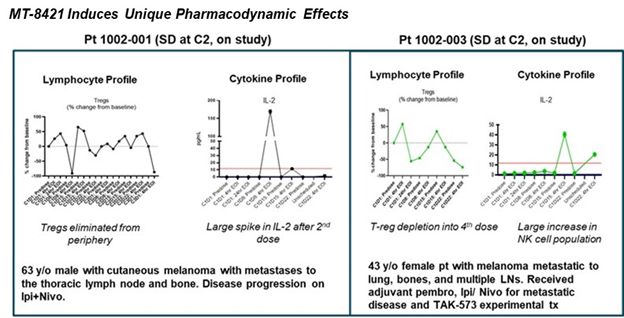
- Three patients were dosed in the first cohort of the phase I study at 32 mcg/kg. No grade 3 or grade 4 drug-related adverse events were observed. Two patients have stable disease and remain on study at cycle 4 and cycle 2, respectively (1 cycle = 4 weeks). One patient had disease progression at the end of cycle 2.
- The two patients in stable disease showed peripheral depletion of Tregs and significant elevations in IL-2 while on therapy.
- Enrollment is on-going in the second cohort of 48 mcg/kg for the phase I study of MT-8421.
MT-0169 (CD38 ETB)
- MT-0169 is designed to destroy CD38+ tumor cells through internalization of CD38 and cell destruction via a novel mechanism of action (enzymatic ribosomal destruction and immunogenic cell death).
- A phase 1 study in patients with relapsed or refractory multiple myeloma was closed on Dec 2023 due to slow patient enrollment in the wake of multiple new approvals in myeloma. This study enrolled 14 patients and no drug-related Grade 4 or 5 adverse events have been observed. One patient with IgA myeloma who was quad-refractory was treated at 5 mcg/kg and had a stringent Complete Response for 16 cycles (1 cycle = 4 weeks) before discontinuing treatment for progression of disease.
- MTEM plans on initiating an investigator sponsored trial with MD Anderson Cancer Center to evaluate MT-0169 in relapsed or refractory CD38+ AML patients.
Research and Collaboration
- MTEM continues to make progress in the drug discovery collaboration with Bristol Myers Squibb.
Previously Announced Process to Explore Strategic Alternatives
Previously, Molecular Templates announced that it has retained Stifel, Nicolaus & Company to assist Molecular Templates in initiating a comprehensive evaluation of strategic alternatives, including, but not limited to, potential financing/recapitalization opportunities, the sale of all, or part, of the company, or a merger, or other strategic transactions. A timetable for completion of this strategic review process has not been set, and there can be no assurance that this strategic review will result in any completed transaction.
About Molecular Templates
Molecular Templates is a clinical-stage biopharmaceutical company focused on the discovery and development of targeted biologic therapeutics. Our proprietary drug platform technology, known as engineered toxin bodies, or ETBs, leverages the resident biology of a genetically engineered form of Shiga-like Toxin A subunit to create novel therapies with potent and differentiated mechanisms of action for cancer.
Forward-Looking Statements
This press release contains forward-looking statements for purposes of the Private Securities Litigation Reform Act of 1995 (the “Act”). Molecular Templates disclaims any intent or obligation to update these forward-looking statements and claims the protection of the Act’s Safe Harbor for forward-looking statements. All statements, other than statements of historical facts, included in this press release, including, but not limited to those regarding strategy, future operations, the Company’s ability to execute on its objectives, prospects, plans, future clinical development of the Company’s product candidates, any implication that the preliminary results, interim results, or the results of earlier clinical trials or ongoing clinical trials will be representative of the results of future or later clinical trials or final results, the potential benefits, safety or efficacy and any evaluations or judgements regarding the Company’s product candidates, [the results of any strategic process which are inherently uncertain at the present time] and future execution of corporate goals. In addition, when or if used in this press release, the words “may,” “could,” “should,” “continue”, “anticipate,” “potential”, “believe,” “estimate,” “appears”, “expect,” “intend,” “plan,” “predict” and similar expressions and their variants, as they relate to Molecular Templates may identify forward-looking statements. Forward-looking statements are not guarantees of future performance and involve risks and uncertainties. Actual events or results may differ materially from those discussed in the forward-looking statements as a result of various factors including, but not limited to the following: the continued availability of financing on commercially reasonable terms, whether Molecular Templates’ cash resources will be sufficient to fund its continuing operations; the results of MTEM’s ongoing clinical studies and its collaboration activities with BMS, the ability to effectively operate MTEM, and those risks identified under the heading “Risk Factors” in Molecular Templates’ filings with the Securities and Exchange Commission (the “SEC”), including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and any subsequent reports filed with the SEC. Any forward-looking statements contained in this press release speak only as of the date hereof, and Molecular Templates specifically disclaims any obligation to update any forward-looking statement, whether because of new information, future events or otherwise.
Contacts:
Grace Kim
grace.kim@mtem.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/82557fa8-2b5b-4168-91a5-86b6398d092c
https://www.globenewswire.com/NewsRoom/AttachmentNg/317e7bb1-dbea-4d97-a9c1-ca10f72615d6








