More Adults With Type 1 Diabetes Achieved A1C Goal (<7%) After Switching From Multiple Daily Insulin Injections or Automated Pumps to Inhaled Insulin (Afrezza®)
Rhea-AI Summary
MannKind (Nasdaq: MNKD) announced positive 30-week results from its Phase 4 INHALE-3 study for Afrezza®, an inhaled insulin for type 1 diabetes. The study showed that more adults achieved target A1c levels (<7%) when switching to or remaining on Afrezza plus basal insulin, compared to usual care (multiple daily injections or insulin pumps).
Key findings include:
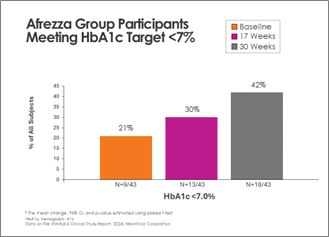
- 100% increase in subjects achieving A1c <7% at 30 weeks in the Afrezza-treated group from baseline
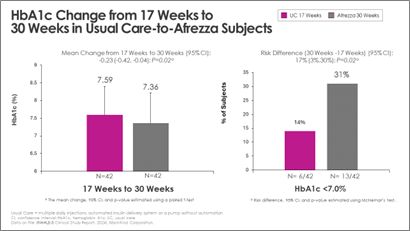
- More than double the subjects achieved A1c <7% at week 30 after switching from usual care to Afrezza at week 17
The company plans to present detailed results at upcoming conferences, including ATTD in March and additional events in 2025.
Positive
- 100% increase in subjects achieving target A1c levels (<7%) at 30 weeks in the Afrezza-treated group
- More than double the subjects achieved target A1c levels after switching from usual care to Afrezza
- Positive data at both 17 and 30 weeks affirms Afrezza as an important option for adult diabetes patients
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, MNKD gained 1.45%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
- 30-week results from the Phase 4 INHALE-3 study expand upon the positive 17-week data presented earlier this year at the American Diabetes Association’s 84th Scientific Sessions
- Switching to, or remaining on Afrezza allowed twice as many people to get to goal during the extension phase
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., Sept. 30, 2024 (GLOBE NEWSWIRE) -- MannKind Corporation (Nasdaq: MNKD), a company focused on the development and commercialization of inhaled therapeutic products and devices for patients with endocrine and orphan lung diseases, today announced top-level 30-week results from its Phase 4 INHALE-3 study, in which additional patients living with type 1 diabetes achieved target A1c levels during the extension phase. The completer analysis which included all people on inhaled insulin, evaluated two separate groups – one that utilized Afrezza® (plus basal insulin) over 30 weeks, and a second group of patients who switched to Afrezza at week 17 from usual care, defined as multiple daily injections (MDI), an automated insulin delivery system, (AID) or a pump without automation.
“The data from the extension phase of this study showed that more people living with T1D are able to reach target A1c levels when they remain on Afrezza (plus basal insulin) or switch to Afrezza from usual care – whether they are using multiple daily injections or pumps,” said Michael Castagna, PharmD, Chief Executive Officer for MannKind Corporation. “We believe this data demonstrates to healthcare practitioners that Afrezza is an effective tool for their patients who want to improve their glycemic control.”
Key Findings:
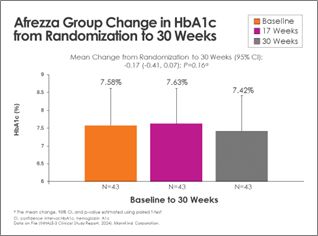
There was continued improvement in the Afrezza (plus degludec)-treated group, with additional subjects achieving A1c <
Switching from usual care to Afrezza (plus degludec) at week-17 allowed more than double the subjects to achieve A1c <
“With the positive data received at both 17- and 30-weeks, we continue to affirm that Afrezza is an important option for adult patients managing their diabetes,” said Dr. Kevin Kaiserman, Senior Vice President, Therapeutic Area Head, Endocrine Diseases for MannKind Corporation. “We look forward to discussing more details of the 30-week study results at ATTD next March and additional conferences in 2025.”
About the INHALE-3 Study
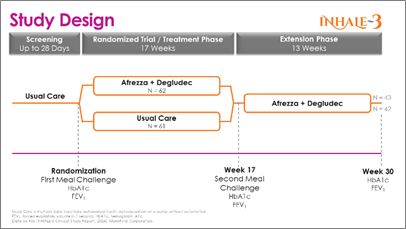
The INHALE-3 study is a 17-week, randomized controlled trial with a 13-week extension conducted across 19 U.S. sites. The study, which enrolled 141 patients (123 randomized), assigned participants over 18 years of age with T1D who are using MDI, an automated insulin delivery system, or a pump without automation to either continue their standard of care or initiate an insulin regimen of a daily basal injection plus Afrezza for boluses (mealtime and corrections). Subjects utilizing Afrezza (inhaled insulin) received a higher initial conversion dose than in the current U.S. product label. Both arms utilized continuous glucose monitoring to assess glucose control.
The randomized control trial (RCT) included an inhaled insulin group that began with 62 subjects at randomization and 57 at 17 weeks; the usual care group consisted of 61subjects at randomization and 58 at 17 weeks. The 17-week results previously shared that the study met its primary efficacy endpoint of a non-inferior change in HbA1c between baseline and week 17 compared to the usual care group. At 17 weeks, those who utilized Afrezza (plus basal insulin) continued with it through the extension phase, and those who were on usual care switched over to Afrezza to week 30. The extension phase started with 45 subjects from the inhaled insulin group and 43 completed the extension; the usual care-to-Afrezza group started with 49 in the extension, with 42 completing. There was no control group in the extension phase. A1c levels were obtained at baseline, 17 and 30-weeks.
More information on the INHALE-3 study is available at: ClinicalTrials.gov(NCT05904743).
About Afrezza
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
Important Safety Information
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and COPD
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
About MannKind
MannKind Corporation (Nasdaq: MNKD) focuses on the development and commercialization of innovative inhaled therapeutic products and devices to address serious unmet medical needs for those living with endocrine and orphan lung diseases.
We are committed to using our formulation capabilities and device engineering prowess to lessen the burden of diseases such as diabetes, nontuberculous mycobacterial (NTM) lung disease, pulmonary fibrosis, and pulmonary hypertension. Our signature technologies – dry-powder formulations and inhalation devices – offer rapid and convenient delivery of medicines to the deep lung where they can exert an effect locally or enter the systemic circulation, depending on the target indication.
With a passionate team of Mannitarians collaborating nationwide, we are on a mission to give people control of their health and the freedom to live life.
Please visit mannkindcorp.com to learn more, and follow us on LinkedIn, Facebook, X or Instagram.
Forward-Looking Statements [to be updated as necessary]
This press release contains forward-looking statements about the planned release of results from a clinical study that involves risks and uncertainties. Words such as “believes”, “anticipates”, “plans”, “expects”, “intends”, “will”, “goal”, “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind’s current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that we may not achieve our projected development goals in the timeframes we expect as well as other risks detailed in MannKind’s filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2023, and subsequent periodic reports on Form 10-Q and current reports on Form 8-K. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
AFREZZA and MANNKIND are registered trademarks of MannKind Corporation.
Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/31d76640-1047-4108-b133-36b48e91775d
https://www.globenewswire.com/NewsRoom/AttachmentNg/650dc7f2-1e72-487b-9047-d0fdce0a795f
https://www.globenewswire.com/NewsRoom/AttachmentNg/040e275d-bd09-4b8c-abff-4b7d76b32321
https://www.globenewswire.com/NewsRoom/AttachmentNg/72bbb5f4-f42f-4a1e-9121-3d4ff8c57790
This press release was published by a CLEAR® Verified individual.









