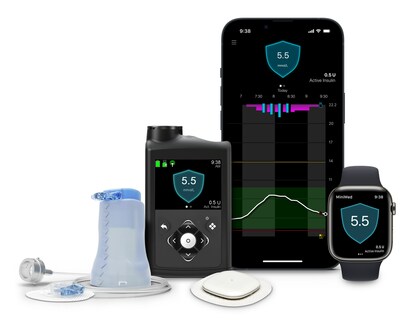
Medtronic Diabetes announces world's first approval for MiniMed™ 780G System with Simplera Sync™ disposable, all-in-one sensor
With CE Mark approval, the benefits of the MiniMed™ 780G system are now available with a new sensor that takes less than 10 seconds to insert1
The MiniMed 780G system with Simplera Sync™ sensor will be available in
The MiniMed™ 780G system is Medtronic's most advanced insulin delivery system, automatically adjusting and correcting† glucose levels every 5 minutes.§ It's the world's only system with a Meal Detection™ feature‡ that is designed to reduce post-meal hyperglycemia when users occasionally forget to give themselves insulin or underestimate the number of carbs in their snacks or meal. The system, which is available with the world's only 7-day infusion set, also features one of the lowest glucose target settings (as low as 100 mg/dL) of any automated insulin delivery system. With this "treat to target" approach, the system more closely mirrors the glucose levels of someone not living with diabetes. With both Simplera Sync™ sensor and the Guardian™ 4 sensor, no fingersticks are required.**
"A challenging aspect of living with diabetes is counting carbohydrates and dosing the right amount of insulin before consuming snacks and meals. Many people underestimate their carbs, which can lead to high blood sugars (hyperglycemia). Prolonged hyperglycemia can lead to serious health problems impacting the eyes, major organs, and even cognitive function, which is particularly concerning in developing children," Robert Vigersky, M.D., Chief Medical Officer, Medtronic Diabetes, Professor of Medicine, Uniformed Services University of the Health Sciences "With its responsive algorithm, the MiniMed™ 780G system can help people living with diabetes even when they occasionally forget to bolus or undercount their carbs. The system takes on more of the work involved in diabetes management and helps alleviate mental burden."
"We're incredibly proud that the MiniMed™ 780G system continues to be the most widely used automated insulin delivery system in
The MiniMed™ 780G system with Simplera Sync™ sensor is indicated for ages 7+ and compatible with iOS and Android. Simplera Sync™ sensor is not approved by the FDA and is limited to investigational use in the U.S.
Simplera Sync™ sensor is designed to leverage Medtronic's advanced AID algorithm as part of its MiniMed™ 780G system while having a similar look and feel as the Simplera™ CGM. The Simplera™ CGM for integrated use with the InPen™ smart insulin pen received CE Mark in September 2023.
About the Diabetes Business at Medtronic (www.medtronicdiabetes.com)
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1. Data on file, CIP330 Evaluation of Updated Continuous Glucose Monitoring (CGM) Form Factor in Adults, Adolescents and Pediatrics.
2. Choudhary, Arrieta, van den Heuvel, Castaneda, Smaniotto, Cohen. Celebrating the data from 100,000 real-world users of the MiniMed 780G system in
*Participants in a clinical trial reported the sensor was smaller and lighter than other sensors they have used. Data on file: CIP330, N=243, ages 2-80.
†Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
‡Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
§ Refers to SmartGuard™ feature. Individual results may vary.
**A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions. Refer to System User Guide – SmartGuard™ feature. Some user interaction required.
Media Kit: https://news.medtronic.com/MiniMed-TM-780G-System-with-Simplera-Sync-TM
Contacts: | |
Ashley Patterson | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-818-576-3025 | +1-763-505-4626 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-diabetes-announces-worlds-first-approval-for-minimed-780g-system-with-simplera-sync-disposable-all-in-one-sensor-302027750.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medtronic-diabetes-announces-worlds-first-approval-for-minimed-780g-system-with-simplera-sync-disposable-all-in-one-sensor-302027750.html
SOURCE Medtronic plc