Immutep and Monash University Announce First Publication Detailing How Human LAG-3 Binds to MHC Class II
Rhea-AI Summary
Immutep (NASDAQ: IMMP) and Monash University have published groundbreaking research in Science Immunology, revealing the first crystal structure of a human LAG-3/HLA-II complex. The study details how human lymphocyte activation gene 3 (LAG-3) binds to its main ligand MHC Class II, providing important insights for developing blocking LAG-3 therapeutics.
The research supports eftilagimod alfa's (efti) mechanism of action through preferential binding to MHC Class II molecules on antigen-presenting cells. The findings, conducted under Professor Jamie Rossjohn at Monash University's Biomedicine Discovery Institute, demonstrate how LAG-3 engages two HLA-II molecules with a distinct 38° offset angle, advancing understanding of the LAG-3 immune control mechanism.
Positive
- First-ever resolution of human LAG-3/HLA-II complex crystal structure
- Findings support development of company's anti-LAG-3 small molecule program
- Research validates eftilagimod alfa's mechanism of action
Negative
- None.
News Market Reaction – IMMP
On the day this news was published, IMMP declined 4.59%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
- Findings published in Science Immunology resolve how human LAG-3 binds to its main ligand providing a better foundation for development of blocking LAG-3 therapeutics, including Immutep’s anti-LAG-3 small molecule program
- Data also supports eftilagimod alfa’s (efti) preferential binding to a subset of MHC Class II molecules on antigen-presenting cells leading to their activation
SYDNEY, AUSTRALIA, Dec. 16, 2024 (GLOBE NEWSWIRE) -- Immutep Limited (ASX: IMM; NASDAQ: IMMP) ("Immutep” or “the Company”), a clinical-stage biotechnology company developing novel LAG-3 immunotherapies for cancer and autoimmune disease, today announces new findings published in Science Immunology that resolve how human lymphocyte activation gene 3 (LAG-3) binds to its main ligand MHC Class II (MHC-II), also known as HLA Class II (HLA-II) in humans. The publication is the first to show the crystal structure of a human LAG-3/HLA-II complex and provides a better foundation for development of blocking LAG-3 therapeutics, including Immutep’s anti-LAG-3 small molecule program.
Under the oversight of Professor Jamie Rossjohn FAA FRS, at Monash University’s Biomedicine Discovery Institute (BDI), and in collaboration with Immutep, this breakthrough is an exemplar of the importance of industry-academia alliances. The LAG-3 immune control mechanism is the exclusive focus of Immutep across both cancer and autoimmunity and a clinically validated target of deep interest throughout the academic, medical, and industry sectors.
Dr. Jan Petersen, first author of the study, said: “The way the PD-1 and CTLA-4 immune checkpoint molecules bind to their respective ligands has been resolved for many years. However, the resolution of the interface between another important checkpoint molecule, LAG-3, and its main ligands, HLA-II molecules, has remained elusive. Solved using data collected at the Australian Synchrotron, a structure of a LAG-3/HLA-II complex provides a structural foundation to harness rationally for future development of antibodies and small molecule therapeutics designed to block LAG-3 activity.”
Dr. Frédéric Triebel, Immutep’s CSO, added: “It is thrilling to be able to see and analyze the interactions taking place at the interface between the soluble homodimeric LAG-3 protein and its main ligand. We now better understand how efti uniquely acts as an MHC-II agonist by preferentially binding to a subset of MHC-II molecules clustered in lipid raft microdomains on the surface of antigen-presenting cells. These findings add to the strong foundation of our work with Professor Rossjohn and his team to develop a deeper understanding of the structure and function of the LAG-3 immune control mechanism, particularly as it relates to our anti-LAG-3 small molecule program.”
The Crystal Structure of the Human LAG-3–HLA-DR1–Peptide Complex publication details how LAG-3 engages two HLA-II molecules (see Figure 1). The data in the publication supports efti’s (soluble LAG-3) preferential binding to a subset of MHC-II molecules on antigen-presenting cells leading to their activation.
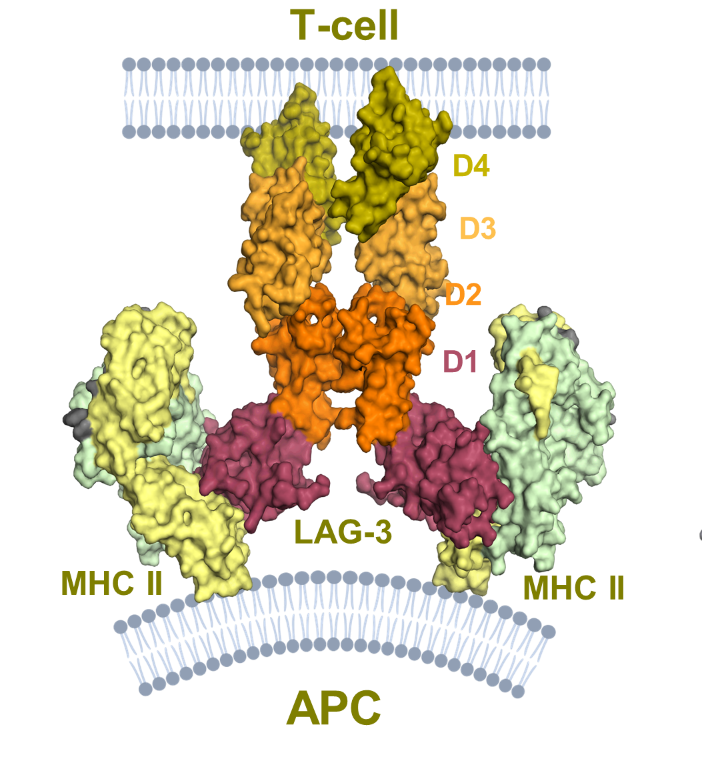
Figure 1: Human LAG-3 homodimer (with domains D1, D2, D3 and D4) binding to two separate HLA-II (MHC-II) molecules on the surface of an antigen-presenting cell (APC), imposing a distinct 38° offset angle. This figure has been modified from the original Figure 1c of Petersen et al to aid visualisation.
About the Monash Biomedicine Discovery Institute
Committed to making the discoveries that will relieve the future burden of disease, the Monash Biomedicine Discovery Institute (BDI) at Monash University brings together more than 120 internationally-renowned research teams. Spanning seven discovery programs across Cancer, Cardiovascular Disease, Development and Stem Cells, Infection, Immunity, Metabolism, Diabetes and Obesity, and Neuroscience, Monash BDI is one of the largest biomedical research institutes in Australia. Our researchers are supported by world-class technology and infrastructure, and partner with industry, clinicians and researchers internationally to enhance lives through discovery.
About Immutep
Immutep is a clinical-stage biotechnology company developing novel LAG-3 immunotherapy for cancer and autoimmune disease. We are pioneers in the understanding and advancement of therapeutics related to Lymphocyte Activation Gene-3 (LAG-3), and our diversified product portfolio harnesses its unique ability to stimulate or suppress the immune response. Immutep is dedicated to leveraging its expertise to bring innovative treatment options to patients in need and to maximise value for shareholders. For more information, please visit www.immutep.com.
Australian Investors/Media:
Catherine Strong, Sodali & Co
+61 (0)406 759 268; catherine.strong@sodali.com
U.S. Media:
Chris Basta, VP, Investor Relations and Corporate Communications
+1 (631) 318 4000; chris.basta@immutep.com
ABN: 90 009 237 889








