Paragon 28®, Inc. Receives FDA Approval for IDE Feasibility Study of Its SMART Total Talus™ System for use in Conjunction with the APEX 3D™ Total Ankle Replacement System
- FDA approval for feasibility study
- Expansion of technology for ankle replacement
- None.
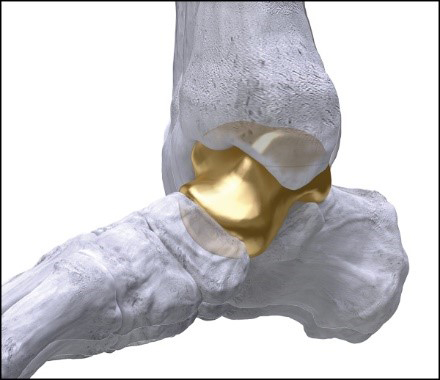
Paragon 28’s first in the world 3D-printed Patient Specific Talus Spacer. Use in treatment of avascular necrosis (AVN). FDA approval: February 17, 2021 through an approval order for a Humanitarian Device Exception (“HDE”). (Graphic: Business Wire)
This milestone is an important achievement in the advancement of Paragon 28’s SMART 28℠ strategy, which targets to provide meaningful options to their surgeon customers and significantly improve patient outcomes. The IDE of the SMART Total Talus adds to the company’s existing Patient Specific Total Talus offering by expanding use of the technology as an option for prospective total ankle replacement candidates.
Paragon 28 acquired Additive Orthopaedics in June 2021, providing the Company with the first and only FDA approved Patient Specific Total Talus replacement for treatment of avascular necrosis (AVN). With approval of the IDE to support future regulatory applications, the company’s SMART Total Talus is on the path to become the only device on the market intended for talar replacement in the setting of adjacent joint arthritis.
Paragon 28’s CEO, Albert DaCosta, commented, “The approval of this IDE study is another great example of our commitment to improve the lives of patients suffering from foot and ankle conditions, and it is an important advancement for our SMART 28 ecosystem and total ankle portfolio. We are very pleased that the FDA has recognized the potential benefits of expanding access of this technology to more ankle patients, and we are excited to get this IDE study underway.”
Paragon 28 extends its gratitude to the FDA for their guidance throughout this IDE approval process and looks forward to further collaboration with the FDA and healthcare professionals and institutions participating in the IDE study.
About Paragon 28, Inc.
Based in
View source version on businesswire.com: https://www.businesswire.com/news/home/20230911941461/en/
Investor Contact:
Matt Brinckman
Senior Vice President, Strategy and Investor Relations
(720) 912-1332
mbrinckman@paragon28.com
Source: Paragon 28, Inc.







