DURECT Announces Peer-Reviewed Article Accepted for Publication with Additional Data from Previously Completed Phase 2a Study of Larsucosterol in Alcohol-Associated Hepatitis
Rhea-AI Summary
DURECT Corporation (NASDAQ: DRRX) announced the acceptance of additional data from its Phase 2a trial evaluating larsucosterol for alcohol-associated hepatitis (AH), which will be published in the American Journal of Gastroenterology. The trial demonstrated a 100% survival rate among 19 patients during a 28-day follow-up, significantly better than the historical mortality rate of 26% in similar patients. The drug was well-tolerated across three doses and showed rapid improvements in liver biomarkers. Furthermore, the company is on track to report data from its ongoing Phase 2b AHFIRM trial in the second half of 2023, targeting 300 patients for further validation of larsucosterol's efficacy. With FDA Fast Track Designation, the company aims to expedite the drug's development to address an urgent medical need, as no FDA-approved therapies currently exist for AH.
Positive
- 100% survival rate (n=19) in the Phase 2a trial at 28 days, compared to a historical rate of 26%.
- Larsucosterol demonstrated rapid improvement in liver biomarkers, including reductions in serum bilirubin levels.
- Well-tolerated across multiple doses with no severe adverse effects reported.
- Ongoing Phase 2b AHFIRM trial with 300 patient enrollment, expected topline data in 2H 2023.
- FDA Fast Track Designation granted to larsucosterol for AH treatment.
Negative
- No FDA-approved therapies currently available for AH, indicating high unmet medical need.
- Results from ongoing and future trials may not replicate previous positive outcomes.
News Market Reaction 1 Alert
On the day this news was published, DRRX gained 0.49%, reflecting a mild positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
Data expands upon previously reported results, including individual patient data, additional liver biomarkers, and comparisons to matching arms from a contemporaneous study
Company on track to report data from ongoing Phase 2b AHFIRM trial in 2H 2023
"The Phase 2a study showed very promising efficacy signals of larsucosterol in AH patients, with all patients surviving the 28-day follow-up period," said
Key results from the article are highlighted below:
- Survival:
100% of patients (n=19) treated with larsucosterol, including 12 patients with severe AH, survived the 28-day follow-up period compared to a historical 28-day mortality rate of26% . - Safety: Larsucosterol was well-tolerated and safe at the three doses studied (30, 90, and 150 mg) when administered as one or two intravenous infusions in subjects with moderate or severe AH. The drug exposure was not affected by the disease severity and was dose proportional.
- Time to discharge:
74% of patients treated with larsucosterol were discharged in under 4 days after a single dose. - Serum bilirubin: Rapid reductions from baseline of serum total bilirubin levels were observed at both Day 7 and Day 28 after larsucosterol dosing, including significant reductions from baseline in moderate AH patients at day 7 and in severe AH patients at day 28.
- Model of End Stage Liver Disease (MELD) Score: Reductions of MELD scores from baseline were observed at Day 7 and Day 28. At Day 28, patients with moderate AH had statistically significantly lower MELD scores at Day 28 and those with severe AH had MELD scores that decreased from baseline at Day 28 but did not achieve statistical significance.
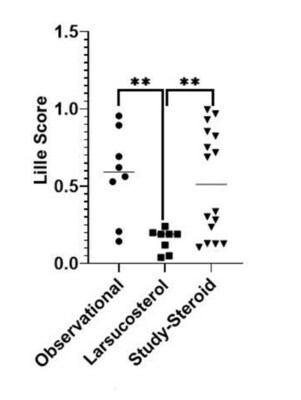
Lille score: All 8 severe AH patients in the 30 or 90 mg dose cohorts were treatment responders (Lille score <0.45) and theirLille scores were statistically lower than those of well-matched patients from the Observational Arm and Study-Steroid Arm of theDASH Consortium trial in a cross-study comparison (see figure).- Liver biomarkers: Both AST and ALT enzymes decreased rapidly in severe AH patients in the 30 or 90mg dose cohorts, with ALT being statistically significantly lower than those in the DASH patients in a cross-study comparison.
About the Larsucosterol AH Phase 2a Trial
Patients with moderate and severe AH were treated with larsucosterol intravenously in this open label, dose escalation,
About the AHFIRM Trial
Enrollment is ongoing in our Phase 2b randomized, double-blind, placebo-controlled, international, multi-center study in subjects with severe acute alcohol-associated hepatitis (AH) to evaluate saFety and effIcacy of laRsucosterol (DUR-928) treatMent (AHFIRM). The study is comprised of three arms targeting enrollment of 300 total patients, with approximately 100 patients in each arm: (1) Placebo plus supportive care, with or without methylprednisolone capsules at the investigators' discretion; (2) larsucosterol (30 mg); and (3) larsucosterol (90 mg). Patients in the larsucosterol arms receive the same supportive care without steroids. In order to maintain blinding, patients in the two active arms receive matching placebo capsules if the investigator prescribes steroids. The primary outcome measure will be the 90-Day incidence of mortality or liver transplantation for patients treated with larsucosterol compared to those treated with placebo.
About Alcohol-associated Hepatitis (AH)
AH is an acute form of alcohol-associated liver disease (ALD), associated with long-term heavy intake of alcohol and often occurs after a recent period of increased alcohol consumption (i.e., a binge). AH is typically characterized by severe inflammation and destruction of liver tissue (i.e., necrosis), potentially leading to life-threatening complications including liver failure, acute kidney injury and multi-organ failure. There are no FDA approved therapies for AH and a retrospective analysis of 77 studies published between 1971 and 2016, which included data from a total of 8,184 patients, showed the overall mortality from AH was
About Larsucosterol (DUR-928)
Larsucosterol is an endogenous sulfated oxysterol and an epigenetic modulator. Epigenetic regulators are compounds that regulate patterns of gene expression without modifying the DNA sequence. DNA hypermethylation, an example of epigenetic dysregulation, results in transcriptomic reprogramming and cellular dysfunction, and is reportedly associated with many acute (e.g., AH) or chronic diseases (e.g., NASH). As an inhibitor of DNA methyltransferases (DNMT1, DNMT3a and 3b), larsucosterol inhibits DNA hypermethylation, which subsequently modulates expression of genes that are involved in cell signaling pathways associated with stress responses, cell death and survival, liver regeneration, and lipid metabolism. This may ultimately lead to improved cell survival, reduced inflammation, and decreased lipotoxicity. As an epigenetic modulator, the proposed mechanism of action provides further scientific rationale for developing larsucosterol for the treatment of certain acute organ injuries and chronic diseases.
About
DURECT is a biopharmaceutical company committed to transforming the treatment of acute organ injury and chronic liver diseases by advancing novel and potentially lifesaving therapies based on its endogenous epigenetic regulator program. Larsucosterol (also known as DUR-928),
DURECT Forward-Looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, relating to: our plans to complete enrollment of the AHFIRM trial in the second quarter of 2023 and report topline data in the second half of 2023, the potential FDA approval of larsucosterol for the treatment of AH, the ability of a positive outcome in the AHFIRM trial to support a New Drug Application filing, the commercialization of POSIMIR by Innocoll, the potential to develop larsucosterol for AH, NASH or other indications, and the potential benefits, if any, of our product candidates. Actual results may differ materially from those contained in the forward-looking statements contained in this press release, and reported results should not be considered as an indication of future performance. The potential risks and uncertainties that could cause actual results to differ from those projected include, among other things, the risks that the AHFIRM trial takes longer to conduct than anticipated due to COVID-19 or other factors, the risk that ongoing and future clinical trials of larsucosterol do not confirm the results from earlier clinical or pre-clinical trials, or do not demonstrate the safety or efficacy of larsucosterol in a statistically significant manner, the risk that the FDA or other government agencies may require additional clinical trials for larsucosterol before approving it for the treatment of AH even if the results of the AHFIRM trial are successful, risks that Innocoll may not commercialize POSIMIR successfully, and risks related to the sufficiency of our cash resources, our anticipated capital requirements, our need or desire for additional financing, our ability to obtain capital to fund our operations and expenses and our ability to continue to operate as a going concern. Further information regarding these and other risks is included in
NOTE: POSIMIR® is a trademark of
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/durect-announces-peer-reviewed-article-accepted-for-publication-with-additional-data-from-previously-completed-phase-2a-study-of-larsucosterol-in-alcohol-associated-hepatitis-301792963.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/durect-announces-peer-reviewed-article-accepted-for-publication-with-additional-data-from-previously-completed-phase-2a-study-of-larsucosterol-in-alcohol-associated-hepatitis-301792963.html
SOURCE