Cybin Reports Positive Topline Data from Phase 2 Study of CYB003 in Major Depressive Disorder with 79% of Patients in Remission after Two 12mg Doses
- Rapid, robust, and clinically significant reduction of depression symptoms observed after a single dose of CYB003, with a clear incremental benefit of a second dose -
- Primary efficacy endpoint achieved with an impressive mean -14 point difference in Montgomery-Asberg Depression Rating Scale (“MADRS”) score reduction from baseline between CYB003 (12mg) vs. placebo (p=0.0005) at 3 weeks -
- At 6 weeks, incremental MADRS score reductions were seen with
- Favorable safety and tolerability profile with no treatment-related serious adverse events at 12mg and 16mg doses -
- Company to host CYB003 Topline Depression Study Review and R&D Briefing today at 10:00am ET in
This news release constitutes a “designated news release” for the purposes of Cybin’s prospectus supplements each dated August 23, 2023, to its short form base shelf prospectus dated August 17, 2023.
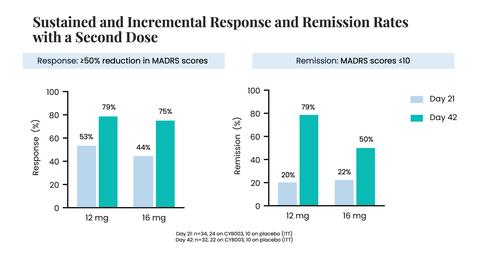
Sustained and Incremental Response and Remission Rates with a Second Dose of CYB003 (Photo: Business Wire)
“We are delighted to share that CYB003 achieved the primary efficacy endpoint in this study and showed rapid and statistically significant improvements in depression symptoms after a single dose, with a clear incremental benefit of a second dose, resulting in 4 out of 5 patients in remission from their depression at 6 weeks,” said Doug Drysdale, Chief Executive Officer of Cybin. “This is an impressive finding and follows on from the unprecedented interim results we announced earlier this month. Importantly, the strength of the data supports progression to a Phase 3 study of CYB003 for the treatment of MDD.”
“The significant reduction in depression symptoms observed in our Phase 2 study is highly gratifying,” said Amir Inamdar, MBBS, DNB (Psych), MFPM, Chief Medical Officer of Cybin. “At the 3-week primary efficacy endpoint, a single 12mg dose of CYB003 showed a rapid, robust, and highly statistically significant improvement in depression symptoms compared to placebo, with a -14.08 point difference in change from baseline in MADRS (p=0.0005, Cohen’s d=2.151). This translated into a very large effect size. Similar significant and robust effects were also seen with a single 16mg dose, which resulted in an improvement in symptoms of depression as measured using the MADRS total score by about 13 points versus placebo. These effects were evident on day one with the 16mg dose and were also highly statistically significant. When data from 12mg and 16mg are pooled, these robust effects are maintained. Further, with two doses, response and remission rates in excess of
Summary of CYB003 Phase 2 topline efficacy data2:
-
Rapid and large improvements in symptoms of depression observed after single doses of CYB003:
- An average of 13.75 points on MADRS (12mg and 16mg cohorts pooled) which is statistically significant from placebo at 3 weeks (p<0.0001) (n=34 total: n=24 on CYB003; n=10 on placebo)
-
Clear incremental benefit of a second dose:
- Incremental and sustained benefit seen as a further 5.8 point improvement on the MADRS total score with a second dose of CYB003 (12mg) at 6 weeks
-
79% of patients were responsive to treatment and79% of patients were in remission from their depression at 6 weeks after receiving two doses of CYB003 (12mg) (n=32 total: n=22 on CYB003; n=10 on placebo)
-
Magnitude of improvement far superior compared to approved antidepressants and recently reported data with other psychedelics:
- Effects translate into unprecedented effect size (Cohen’s d > 2) (SSRIs = 0.243)
-
These results compare favorably to pooled data from 232 industry studies of current standard of care antidepressants, selective serotonin reuptake inhibitors (“SSRIs”), submitted to
U.S. Food and Drug Administration (“FDA”) which show an average improvement of 1.82 points on the MADRS total score vs. placebo.3
- Data support progression to a Phase 3 study
Safety and tolerability:
- CYB003 has demonstrated an excellent safety profile in doses tested, with all reported adverse events mild to moderate and self–limiting.
“Completing our Phase 2 study and presenting this extraordinary topline safety and efficacy data represents a much-anticipated milestone for us – a goal that we have all been tirelessly working toward. Our objective remains to design and deliver improved treatments for those suffering with mental health disorders, and we believe that we are one step closer to achieving that goal,” concluded Drysdale.
Upcoming Milestones for the CYB003 Program4
Cybin plans to submit topline data to the
Phase 2 Topline Study Review and R&D Briefing to be held today at 10:00 a.m. ET in
Cybin’s leadership team is hosting a Phase 2 Topline study review and R&D briefing event today, featuring Dr. Maurizio Fava, MD, Massachusetts General Hospital & Harvard Medical School, and Dr. Gitte Moos Knudsen, Professor, Chief Neurologist, DMSc, Neurobiology Research Unit, Rigshospitalet and University of
Click here to register for the live webcast. A replay of the event will also be available on the Company’s investor relations website on the Events & Presentations page.
About Cybin
Cybin is a clinical-stage biopharmaceutical company on a mission to create safe and effective psychedelic-based therapeutics to address the large unmet need for new and innovative treatment options for people who suffer from mental health conditions.
Cybin’s goal of revolutionizing mental healthcare is supported by a network of world-class partners and internationally recognized scientists aimed at progressing proprietary drug discovery platforms, innovative drug delivery systems, and novel formulation approaches and treatment regimens. The Company is currently developing CYB003, a proprietary deuterated psilocybin analog for the treatment of major depressive disorder and CYB004, a proprietary deuterated DMT molecule for generalized anxiety disorder and has a research pipeline of investigational psychedelic-based compounds.
Headquartered in
Footnotes
- A p-value indicates statistical significance. Values <0.05 are considered statistically significant and values <0.001 are considered highly statistically significant. Cohen’s d represents size of the effect. An effect size of 2.15 is considered large.
- All data are preliminary.
- Stone et al, 2022
- The advancement of Cybin’s CYB003 program is contingent on Cybin’s ability to continue raising capital under its current and future financing arrangements. No assurances can be given that Cybin will be able to raise the additional capital that it may require for its anticipated future development.
Cautionary Notes and Forward-Looking Statements
Certain statements in this news release relating to the Company are forward-looking statements and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe” or “continue”, or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding the Cybin’s plans to report CYB003 12-week durability data in Q1 2024; progression to Phase 3 development of CYB003 in early 2024; recruitment for a CYB003 Phase 3 study and commencement of the Phase 3 study in late Q1 2024; the Company’s plan to request an end of Phase 2 meeting with the FDA in early 2024; and the Company’s proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health disorders.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the spread of COVID-19 on the Company's operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in each of the Company's management's discussion and analysis for the three and six month periods ended September 30, 2023, and the Company’s annual information form for the year ended March 31, 2023, which are available under the Company's profile on www.sedarplus.ca and with the
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The
Neither the Neo Exchange Inc. nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231130470838/en/
Investor & Media:
Gabriel Fahel
Chief Legal Officer
Cybin Inc.
1-866-292-4601
irteam@cybin.com – or – media@cybin.com
Source: Cybin Inc.






