CEL-SCI’s Phase 3 Population Analysis for Upcoming Confirmatory Registration Study in Head & Neck Cancer Demonstrates Well Balanced Patient Population, Confidence in Clinical Results
- Multikine, a true first-line cancer immunotherapy, cut the 5-year risk of death by half compared to control in its target population
- Bias analysis conducted in preparation for submission of data to regulatory agencies including the FDA for confirmatory registration study
- Detailed data on parameters including patient age, sex, race, tumor locations, and staging demonstrate balance between the treatment and control arms, no bias found, supporting confidence in Multikine’s efficacy results
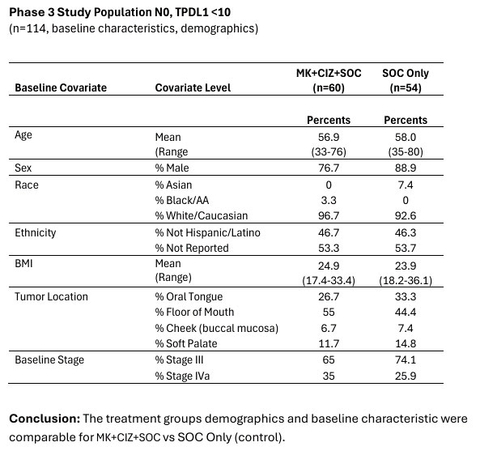
The above table includes detailed results from the bias analysis involving the 2 groups, treated vs. control (Graphic: Business Wire)
CEL-SCI’s bias analysis concluded that the treatment group demographics and baseline characteristics were comparable for the Multikine treated and control arms of the Phase 3 study. There were no confounding baseline parameters in the Multikine-treated versus control population. No bias was present in the study and none was detected in favor of the investigational product, Multikine. As such, the study data are reliably interpretable, statistically significant and have been shown to support the clinical effect of neoadjuvant (pre-surgery) Multikine immunotherapy in extending the life of these patients in the Phase 3 study.
This is critically important information because CEL-SCI, with the FDA’s agreement, will be conducting a 212-patient confirmatory registration study for Multikine. The target population for the confirmatory study shows a 5-year survival in advanced primary head and neck cancer of
“Multikine’s efficacy results in the target population are truly impressive and the bias analysis is critical to demonstrate a high degree of confidence in the data. A hazard ratio of 0.35 with an upper limit of 0.66 is excellent and suggests a very high chance to repeat the great survival benefit from Multikine. The bias analysis showed that the survival benefit is truly from Multikine as opposed to an imbalance in the patient population,” stated CEL-SCI CEO Geert Kersten. “We are pleased to share the baseline results as we prepare to submit the data to regulators ahead of our upcoming confirmatory registration study.”
The bias analysis was conducted for the entire Phase 3 study population of 923 patients with newly diagnosed resectable, locally advanced primary head and neck cancer, as well as the subgroup of 114 patients who had no lymph node involvement and had low PD-L1 tumor expression (determined via biopsy), the target population for CEL-SCI’s upcoming confirmatory registration study.
About CEL-SCI Corporation
CEL-SCI believes that boosting a patient’s immune system while it is still intact should provide the greatest possible impact on survival. Multikine is designed to help the immune system "target" the tumor at a time when the immune system is still relatively intact and thereby thought to be better able to mount an attack on the tumor.
Multikine (Leukocyte Interleukin, Injection), a true first-line cancer therapy, has been dosed in over 750 patients and received Orphan Drug designation from the FDA for neoadjuvant therapy in patients with squamous cell carcinoma (cancer) of the head and neck. Multikine significantly extended life in its target patient population demonstrating a
The Company has operations in
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. When used in this press release, the words "intends," "believes," "anticipated," "plans" and "expects," and similar expressions, are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such statements include, but are not limited to, statements about the terms, expected proceeds, use of proceeds and closing of the offering. Factors that could cause or contribute to such differences include an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI's filings with the Securities and Exchange Commission, including but not limited to its report on Form 10-K for the year ended September 30, 2023. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
* Multikine (Leukocyte Interleukin, Injection) is the trademark that CEL-SCI has registered for this investigational therapy. This proprietary name is subject to FDA review in connection with the Company's future anticipated regulatory submission for approval. Multikine has not been licensed or approved for sale, barter or exchange by the FDA or any other regulatory agency. Similarly, its safety or efficacy has not been established for any use.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240726306579/en/
Gavin de Windt
CEL-SCI Corporation
(703) 506-9460
Source: CEL-SCI Corporation









