New Broad-Spectrum Antiviral Currently in a Phase 2a Clinical Trial in Influenza A may be Effective Against the H5N1 Avian Influenza Strain Identified in Humans Exposed to Infected Dairy Cows
Rhea-AI Summary
Cocrystal Pharma (Nasdaq: COCP) has announced that its novel antiviral, CC-42344, shows promise against the highly pathogenic avian influenza A (H5N1) strain. Recent in vitro studies indicate that CC-42344 inhibits the influenza A virus's PB2 protein, essential for replication and transcription. The drug is currently in a Phase 2a clinical trial assessing its safety, tolerability, and antiviral benefits in influenza A patients, with top-line results expected in late 2024. Previously, a Phase 1 study in healthy volunteers demonstrated favorable safety and tolerability. The company used its proprietary structure-based platform to create a high-resolution crystal structure of the avian PB2 protein, confirming CC-42344's binding efficacy. The CDC has reported recent avian flu outbreaks in the U.S., including infections in dairy cows affecting humans.
Positive
- CC-42344 shows potential efficacy against the highly pathogenic avian influenza A (H5N1) strain.
- CC-42344 is currently in a Phase 2a clinical trial, with results expected in the second half of 2024.
- Previous Phase 1 study demonstrated favorable safety and tolerability.
- The drug binds to a highly conserved region of the avian influenza A H5N1 PB2 protein, essential for viral replication.
Negative
- The effectiveness of CC-42344 is still under clinical trial, with no confirmed results yet.
- The avian flu has been reported in more than 100 dairy herds across 12 U.S. states, posing ongoing risks.
News Market Reaction 1 Alert
On the day this news was published, COCP gained 2.41%, reflecting a moderate positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
BOTHELL, Wash., June 20, 2024 (GLOBE NEWSWIRE) -- Cocrystal Pharma, Inc.’s (Nasdaq: COCP) novel, broad-spectrum antiviral CC-42344 inhibits activity in the highly pathogenic avian influenza A (H5N1) PB2 protein recently identified in infected dairy cattle, according to recently completed in vitro studies. CC-42344 is a new class of antiviral drugs designed to block essential steps in the replication and transcription of the influenza A virus.
Cocrystal is conducting an influenza A Phase 2a clinical study with orally administered CC-42344 and expects to report topline results in the second half of 2024. This study is evaluating the safety, tolerability, antiviral and clinical benefits in influenza A infected subjects. In late 2022 Cocrystal reported favorable safety and tolerability results from a Phase 1 study in healthy volunteers.
Cocrystal demonstrated the potential efficacy of CC-42344 against the new avian flu strain with the recently published genome sequence for H5N1. Using its proprietary structure-based platform, Cocrystal created a high-resolution crystal structure of this avian PB2 protein and confirmed that CC-42344 binds to its highly conserved PB2 region. The in vitro data were generated testing CC-42344 against the avian H5N1 PB2 protein and further support CC-42344’s activity similar to that of Cocrystal’s data with pandemic and seasonal influenza A.
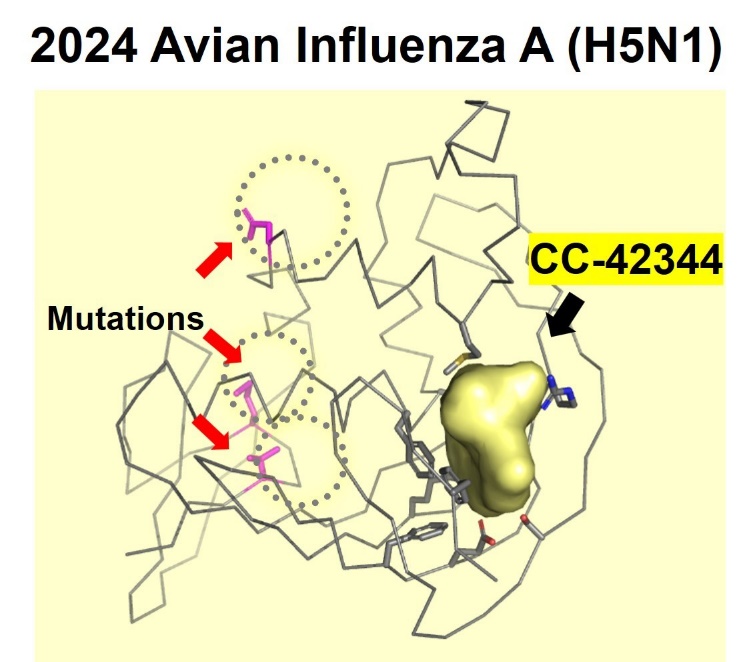
CC-42344 binds to the highly conserved region of
the avian influenza A H5N1 PB2 protein
“The findings validate our broad-spectrum approach to the treatment and prevention of pandemic flu. This is important as there are no specific FDA-approved vaccines to prevent infections by this virus in humans,” said Sam Lee, PhD, President and co-CEO of Cocrystal. “These findings support our previously reported preclinical data showing that CC-42344 is highly active against seasonal and pandemic influenza A strains, including emerging mutations. CC-42344 is an inhibitor compound providing a unique mechanism of action with a high barrier to resistance.”
“Recent CDC reported avian flu outbreaks in the U.S., which include the first cases of humans exposed to infected dairy cows, are concerning,” said James Martin, CFO and co-CEO of Cocrystal. “The CDC reported three additional cases of avian influenza infection from exposure to dairy cows in early June and avian flu is now confirmed in more than 100 dairy herds in 12 U.S. states.”
About Avian Influenza A H5N1
Avian influenza A H5N1 was reported in 889 cases and caused 463 deaths in 23 countries between 2003 and April 2024, according to the World Health Organization (WHO). On April 1, 2024, the CDC reported a case of highly pathogenic avian influenza A H5N1 in a farmworker in Texas during a multistate outbreak of avian influenza in dairy cows. Two more cases were subsequently reported in farmworkers in Michigan.
The CDC analyzed sera (blood) collected from people of all ages in all 10 Health & Human Services regions during the 2022-2023 and 2021-2022 flu seasons. These samples were challenged with H5N1 virus to see whether there was an antibody reaction. Data from this study suggest that there is extremely low to no population immunity to clade 2.3.4.4b A (H5N1) viruses in the U.S. Antibody levels remained low regardless of whether or not the participants received a seasonal flu vaccination, meaning that seasonal flu vaccination did not produce antibodies to H5N1 viruses.
Cocrystal Pharma determined the high resolution X-ray crystal structure of the recent avian influenza A (H5N1) PB2 protein and confirmed activity of CC-42344 in vitro (NIH GeneBank ID:influenza A/Texas/37/2024(H5N1). The crystal structure of the avian influenza A (H5N1) PB2 protein showed new mutations located outside the PB2 active site. Subsequent studies showed that CC-42344 binds to the active site of the avian influenza A (H5N1) PB2 protein as previously demonstrated with the pandemic and seasonal influenza A PB2. Preliminary in vitro assays confirmed that CC-42344 exhibits high potency against the avian influenza A (H5N1) PB2 protein.
About CC-42344
CC-42344 is Cocrystal’s novel, broad-spectrum, antiviral investigational candidate for the treatment of pandemic and seasonal influenza A. CC-42344 inhibits the first step in influenza A’s viral replication by binding to a highly conserved PB2 site of the influenza polymerase complex that is essential to replication and was discovered using Cocrystal’s proprietary structure-based drug discovery platform technology.
Cocrystal is conducting a Phase 2a human challenge study in the United Kingdom to evaluate safety, viral and clinical measures of oral CC-42344 in healthy volunteers who are challenged with influenza A. CC-42344 was advanced into Phase 2a testing following favorable safety and tolerability results reported in a Phase 1 study in healthy volunteers conducted in Australia. In vitro testing showed CC-42344’s excellent antiviral activity against influenza A strains, including pandemic and seasonal strains, as well as against strains resistant to Tamiflu® and Xofluza®, while also demonstrating favorable pharmacokinetic and safety profiles.
About Cocrystal Pharma, Inc.
Cocrystal Pharma, Inc. is a clinical-stage biotechnology company discovering and developing novel antiviral therapeutics that target the replication process of influenza viruses, coronaviruses (including SARS-CoV-2), noroviruses and hepatitis C viruses. Cocrystal employs unique structure-based technologies and Nobel Prize-winning expertise to create first- and best-in-class antiviral drugs. For further information about Cocrystal, please visit www.cocrystalpharma.com.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding the potential efficacy of CC-42344 against the avian influenza A H5N1 virus, the expected timing and results of the Phase 2a trial for CC-42344 for the oral treatment of influenza A in 2024, and the potential market for such product candidate. The words "believe," "may," "estimate," "continue," "anticipate," "intend," "should," "plan," "could," "target," "potential," "is likely," "will," "expect" and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events. Some or all of the events anticipated by these forward-looking statements may not occur. Important factors that could cause actual results to differ from those in the forward-looking statements include, but are not limited to, risks relating to our ability to obtain regulatory authority for and proceed with clinical trials including recruiting volunteers and procuring materials for such studies by our clinical research organizations and vendors, the results of such studies, our and our collaboration partners’ technology and software performing as expected, general risks arising from clinical studies, receipt of regulatory approvals, regulatory changes, and potential development of effective treatments and/or vaccines by competitors, including as part of the programs financed by the U.S. government, potential mutations in a virus we are targeting that may result in variants that are resistant to a product candidate we develop. Further information on our risk factors is contained in our filings with the SEC, including our Annual Report on Form 10-K for the year ended December 31, 2023. Any forward-looking statement made by us herein speaks only as of the date on which it is made. Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future developments or otherwise, except as may be required by law.
Investor Contact:
LHA Investor Relations
Jody Cain
310-691-7100
jcain@lhai.com
Media Contact:
JQA Partners
Jules Abraham
917-885-7378
Jabraham@jqapartners.com
# # #









