Biohaven Real-World Study Highlights Increased Healthcare Utilization Among Americans with Episodic Migraine having Higher Levels of Migraine-Related Disability
Rhea-AI Summary
Biohaven Pharmaceutical Holding Company (NYSE: BHVN) published research indicating a significant correlation between migraine-related disability and increased healthcare resource utilization among Americans with episodic migraines. The study revealed that over 25% of participants reported severe disability, leading to higher medical costs. These results, published in the March issue of Headache: The Journal of Head and Face Pain, suggest that targeting severely disabled patients with effective treatments could lower costs and improve patient care.
Positive
- Publication of research linking migraine-related disability to healthcare utilization.
- Potential for cost-effective interventions targeting high disability patients.
Negative
- 26% of patients reported severe or very severe migraine-related disability.
- Higher disability levels lead to increased medical and pharmacy costs.
News Market Reaction
On the day this news was published, BHVN declined 5.74%, reflecting a notable negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
- More than
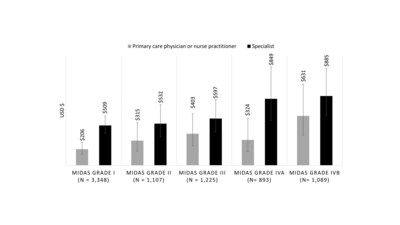
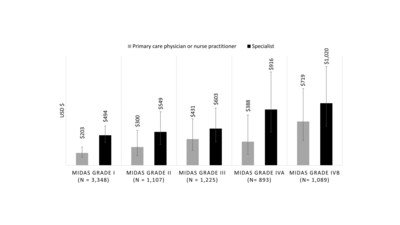
25% of patients reported being severely or very severely impacted by their migraine attacks - Study showed higher levels of migraine-related disability were associated with increased HCRU costs in primary care and specialty settings among adults with episodic migraine
- Data suggests that assessment of disability in people with migraine may support prescribing of cost-effective interventions, particularly among those with severe disability
NEW HAVEN, Conn., April 14, 2022 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) announced the publication of new real-world research showing that as migraine-related disability increases, healthcare utilization also increases among Americans with episodic migraine. Targeting high disability patients with effective treatments may reduce disability and improve the cost-effectiveness of medical care among primary care and specialty providers. These findings were published in the March issue of Headache: The Journal of Head and Face Pain, the official journal of the American Headache Society.
Participants in this retrospective, cohort study were identified using claims and electronic health record data from the Decision Resources Group database. Adults (aged ≥18 years) were eligible if they had received a diagnosis of migraine with or without aura, as defined by International Classification of Disease codes (ICD-9 or ICD-10) and completed a Migraine Disability Assessment Scale (MIDAS) questionnaire between January 2016 and December 2018. Researchers explored the associations between migraine-related disability, as measured by MIDAS score, and the cost of HCRU for the 6 months after MIDAS assessment in primary care and specialty settings.
One of the important findings of the study is that among the 7,662 adults who were analyzed,
"This study describes the relationships between MIDAS grades, healthcare resource utilization, and direct medical costs," said Richard Lipton, M.D., professor and vice chair of Neurology at Albert Einstein College of Medicine, who developed the MIDAS instrument and is senior author on the study. "Our findings suggest that disability assessments in people with migraine may be used to target individuals with the most to gain from effective treatment. The cost offsets of effective treatment should be assessed in these most disabled and costly patient groups."
Gil L'Italien Ph.D., Senior Vice President, GHEOR & Epidemiology, Biohaven, and co-author of the study, observed, "This analysis, derived from claims and health record data representing
About Migraine
Nearly 40 million people in the U.S. suffer from migraine and the World Health Organization classifies migraine as one of the 10 most disabling medical illnesses. Migraine is characterized by debilitating attacks lasting four to 72 hours with multiple symptoms, including pulsating headaches of moderate to severe pain intensity that can be associated with nausea or vomiting, and/or sensitivity to sound (phonophobia) and sensitivity to light (photophobia). There is a significant unmet need for new treatments as more than 90 percent of people with migraine are unable to work or function normally during an attack.
About Biohaven
Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's Neuroinnovation™ portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute and preventive treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, and spinocerebellar ataxia; MPO inhibition for amyotrophic lateral sclerosis; Kv7 Ion Channel Activators (Kv7), and Myostatin. More information about Biohaven is available at www.biohavenpharma.com and Nurtec ODT at www.nurtec.com.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements regarding the ability to commercialize NURTEC®️ ODT (rimegepant), delays or problems in the supply or manufacture of commercial product, complying with applicable U.S. regulatory requirements, the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials, the timing of planned interactions with the FDA, the timing and outcome of regulatory filings, the potential commercialization of Biohaven's product candidates, the potential for Biohaven's product candidates to be first in class or best in class therapies and the effectiveness and safety of Biohaven's product candidates. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements. Additional important factors to be considered in connection with forward-looking statements are described in the "Risk Factors" section of Biohaven's Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on February 25, 2022, and Biohaven's subsequent filings with the Securities and Exchange Commission. The forward-looking statements are made as of this date and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. Neuroinnovation is a trademark of Biohaven Pharmaceutical Holding Company Ltd.
Biohaven Contact
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
201-248-0741
Media Contact
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
312-961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-real-world-study-highlights-increased-healthcare-utilization-among-americans-with-episodic-migraine-having-higher-levels-of-migraine-related-disability-301525370.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-real-world-study-highlights-increased-healthcare-utilization-among-americans-with-episodic-migraine-having-higher-levels-of-migraine-related-disability-301525370.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.