BD Announces Expanded 510(k) Clearance for At-Home System for Ascites
Rhea-AI Summary
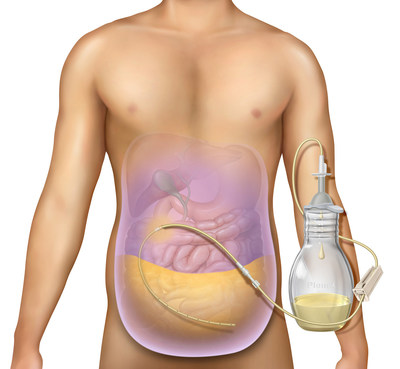
BD (Becton, Dickinson and Company) announced it received 510(k) clearance from the FDA for its PeritX™ Peritoneal Catheter System to drain symptomatic, recurrent non-malignant ascites. This system, which expands on the existing PleurX™ system, allows patients to manage their condition at home, reducing hospital visits. The PeritX™ is now the first FDA-approved tunneled catheter for both malignant and non-malignant ascites, improving patient care and comfort.
Positive
- Received FDA 510(k) clearance for PeritX™ Peritoneal Catheter System.
- First FDA indicated tunneled catheter for both malignant and non-malignant ascites.
- Allows at-home management of ascites, reducing hospital visits.
Negative
- None.
News Market Reaction 1 Alert
On the day this news was published, BDX declined 0.04%, reflecting a mild negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
FRANKLIN LAKES, N.J., June 23, 2021 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the PeritX™ Peritoneal Catheter System for the drainage of symptomatic, recurrent non-malignant ascites. Ascites is a debilitating condition that causes the build-up of fluid in the abdomen.
The PleurX™ Peritoneal Catheter System was introduced in 2005 for the drainage of malignant ascites. With the new, expanded indication, the system is being rebranded as the PeritX™ Peritoneal Catheter System to make it easier for physicians to navigate BD's portfolio of at-home drainage products. The PeritX™ Peritoneal Catheter System is the first and only FDA indicated tunneled catheter for the drainage of malignant and non-malignant ascites and for the palliation of symptoms related to recurrent ascites.
"The new, expanded indication for the PeritX™ Peritoneal Catheter System will allow us to help more patients who present with ascites due to liver disease or other noncancer-related origins," said Padraic O'Brien, Worldwide President of Peripheral Intervention for BD. "Recurrent ascites is a debilitating condition that requires the patient to make frequent trips to the hospital so that fluid can be removed from the peritoneum. The PeritX™ Peritoneal Catheter System allows patients to manage non-malignant ascites at home and aligns with our goal to help keep patients out of the hospital."
Ascites is a condition in which fluid collects in spaces within the abdomen. It can make ordinary daily activities more challenging. The fluid may also move to the chest and surround the lungs, making breathing more difficult.
"Patients with ascites are often managing multiple serious medical conditions. The PeritX™ Peritoneal Catheter System provides relief from the discomfort of ascites fluid build-up, at home, enabling patients to spend more time with their loved ones," O'Brien concluded.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media | Investors |
Paul Tyahla | Kristen M. Stewart, CFA |
BD Public Relations | BD SVP, Strategy & Investor Relations |
908.873.4412 | 201.847.5378 |
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/bd-announces-expanded-510k-clearance-for-at-home-system-for-ascites-301317926.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/bd-announces-expanded-510k-clearance-for-at-home-system-for-ascites-301317926.html
SOURCE BD (Becton, Dickinson and Company)