Alexandria to lead mission-critical panel on immunology and inflammation as a cornerstone of treating human disease tomorrow at the 2024 Galien Forum USA at the Alexandria Center® for Life Science – New York City
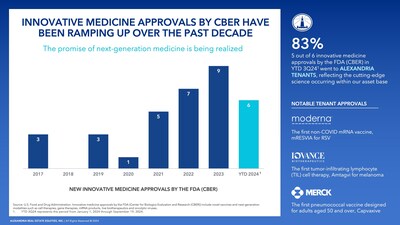
PASADENA, Calif., Nov. 6, 2024 /PRNewswire/ -- Alexandria Real Estate Equities, Inc. (NYSE: ARE), the first, preeminent, longest-tenured and pioneering owner, operator and developer of collaborative mega campuses in AAA life science innovation cluster locations, continues to leverage its position at the vanguard and heart of the $5 trillion secularly growing life science industry to foster the development of new therapies and cures for the 10,000 diseases known to humankind, of which less than 10% are currently addressable with treatments. With $4.5 trillion in U.S. healthcare spending in 2022, the majority of which is attributed to hospital and physician services, the opportunity to reduce the economic burden on the country and better manage disease for patients through the development of new innovative medicines remains immense. Driven by decades of scientific discovery, the U.S. Food and Drug Administration (FDA) this year has approved 38 novel small molecule and biologic therapies, as well as six new innovative medicines, which include novel vaccines and next-generation modalities such as cell, gene and mRNA-based therapies. Alexandria tenants are responsible for five of these six innovative medicines, reflecting the cutting-edge science taking place within the company's Labspace® facilities and the high quality of the company's client base. Innovative medicines continue to gain momentum with a 286% increase in approvals by the FDA in this class between the 2017–2020 and 2021–YTD 2024 periods.
"As innovative medicine approvals by the FDA have ramped up over the past several years, the promise of next-generation medicine is being realized in key areas of unmet need, including oncology, neurodegenerative and psychiatric disorders and diseases of the immune system, which means expanded treatment options and enhanced quality of life for patients," said Joel S. Marcus, executive chairman and founder of Alexandria Real Estate Equities, Inc. and Alexandria Venture Investments. "Innovation in medicine is the only effective path to solving the over 90% of diseases that currently have no treatments and to reducing long-term costs associated with the healthcare system. New medicines delivered to patients and early detection of costly diseases, like Alzheimer's or cancer, mean fewer visits to the hospital and fewer treatments over the long term. At Alexandria, we are working to ensure that the life science industry can maintain its collaborative science-driven advantage as one of the world's most innovative, impactful and critical industries, and one that is vastly improving health and well-being."
Alexandria has a remarkable track record of partnering with trailblazing life science companies to enable the development of life-changing and lifesaving treatments and cures. Since 2013, Alexandria tenants have developed or commercialized half the novel FDA-approved therapies, and so far in 2024, they have been responsible for over 80% of the FDA approvals for innovative medicines. Many of these approvals are first-of-their-kind medicines. Notably, Moderna, with which Alexandria began its strategic relationship in 2012, received approval for a respiratory syncytial virus (RSV) vaccine. Moderna's novel RSV vaccine validates the potential of its paradigm-shifting mRNA technology to address multiple diseases, and it is also the first mRNA vaccine to be approved for a disease other than COVID-19. Included among the Alexandria tenants harnessing the immune system to treat cancer is Iovance Biotherapeutics, which received FDA approval for the first tumor-infiltrating lymphocyte cell therapy to treat advanced melanoma. Alexandria has been providing mission-critical real estate to Iovance in the San Francisco Bay Area since early 2022.
The field of immunology and inflammation has emerged as fundamental to how we approach a wide array of human diseases. Recent breakthroughs in our understanding of the immune system are leading to revolutionary therapies and opening new frontiers in medical science. Moreover, immunology is predicted to become the second-largest area of biopharmaceutical spend by 2028, behind oncology, and the global immunology market is expected to more than double from nearly $103 billion in 2024 to $257 billion by 2032.
In light of the paramount importance of this burgeoning field, Alexandria is leading a mission-critical panel titled "Immunology & Inflammation – A Cornerstone of Treating Human Disease" tomorrow, November 7, 2024, at the 2024 Galien Forum USA at the Alexandria Center® for Life Science – New York City. Co-moderated by Mr. Marcus and Lynne Zydowsky, PhD, chief of science of Alexandria, the pivotal discussion will feature pioneers in immunology who will delve into the latest advances, current challenges and future opportunities for harnessing the immune system to combat chronic and inflammatory disorders. Alexandria's panel will also explore how immunological and anti-inflammatory approaches are reshaping our understanding of disease mechanisms and opening new opportunities for treatment. These latest developments — from engineered cell therapies to novel immunomodulatory drugs — hold the promise to transform the treatment landscape for millions of patients worldwide.
About Alexandria Real Estate Equities, Inc.
Alexandria Real Estate Equities, Inc. (NYSE: ARE), an S&P 500® company, is a best-in-class, mission-driven life science REIT making a positive and lasting impact on the world. As the pioneer of the life science real estate niche with our founding in 1994, Alexandria is the preeminent and longest-tenured owner, operator and developer of collaborative mega campuses in AAA life science innovation cluster locations, including Greater Boston, the San Francisco Bay Area, San Diego, Seattle, Maryland, Research Triangle and New York City. As of September 30, 2024, Alexandria has a total market capitalization of $33.1 billion and an asset base in North America that includes 41.8 million RSF of operating properties, 5.3 million RSF of Class A/A+ properties undergoing construction, and one committed near-term project expected to commence construction in the next two years. Alexandria has a longstanding and proven track record of developing Class A/A+ properties clustered in mega campuses that provide our innovative tenants with highly dynamic and collaborative environments that enhance their ability to successfully recruit and retain world-class talent and inspire productivity, efficiency, creativity and success. Alexandria also provides strategic capital to transformative life science companies through our venture capital platform. We believe our unique business model and diligent underwriting ensure a high-quality and diverse tenant base that results in higher occupancy levels, longer lease terms, higher rental income, higher returns and greater long-term asset value. For more information on Alexandria, please visit www.are.com.
Forward-Looking Statements
This press release includes "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such forward-looking statements include, without limitation, statements regarding Alexandria's impact on the life science industry, on its tenants' business and pursuit of novel treatment and cures and on human health and well-being. These forward-looking statements are based on Alexandria's present intent, beliefs or expectations, but forward-looking statements are not guaranteed to occur and may not occur. Actual results may differ materially from those contained in or implied by Alexandria's forward-looking statements as a result of a variety of factors, including, without limitation, the risks and uncertainties detailed in its filings with the Securities and Exchange Commission. All forward-looking statements are made as of the date of this press release, and Alexandria assumes no obligation to update this information. For more discussion relating to risks and uncertainties that could cause actual results to differ materially from those anticipated in Alexandria's forward-looking statements, and risks and uncertainties to Alexandria's business in general, please refer to Alexandria's filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and any subsequently filed quarterly reports on Form 10-Q.
CONTACT: Joel S. Marcus, Executive Chairman & Founder, (626) 578-9693, jmarcus@are.com
 View original content to download multimedia:https://www.prnewswire.com/news-releases/alexandria-real-estate-equities-inc-enables-the-discovery-development-and-delivery-of-new-innovative-medicines-that-are-key-to-addressing-significant-unmet-medical-need-302296686.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/alexandria-real-estate-equities-inc-enables-the-discovery-development-and-delivery-of-new-innovative-medicines-that-are-key-to-addressing-significant-unmet-medical-need-302296686.html
SOURCE Alexandria Real Estate Equities, Inc.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/alexandria-real-estate-equities-inc-enables-the-discovery-development-and-delivery-of-new-innovative-medicines-that-are-key-to-addressing-significant-unmet-medical-need-302296686.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/alexandria-real-estate-equities-inc-enables-the-discovery-development-and-delivery-of-new-innovative-medicines-that-are-key-to-addressing-significant-unmet-medical-need-302296686.html








