SYFOVRE® (pegcetacoplan injection) Preserved Visual Function at 36 Months in GALE Extension Study in Geographic Atrophy (GA)
Rhea-AI Summary
Apellis Pharmaceuticals announced that their SYFOVRE® (pegcetacoplan injection) has shown to preserve visual function for 36 months in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD), as revealed in the GALE long-term extension study.
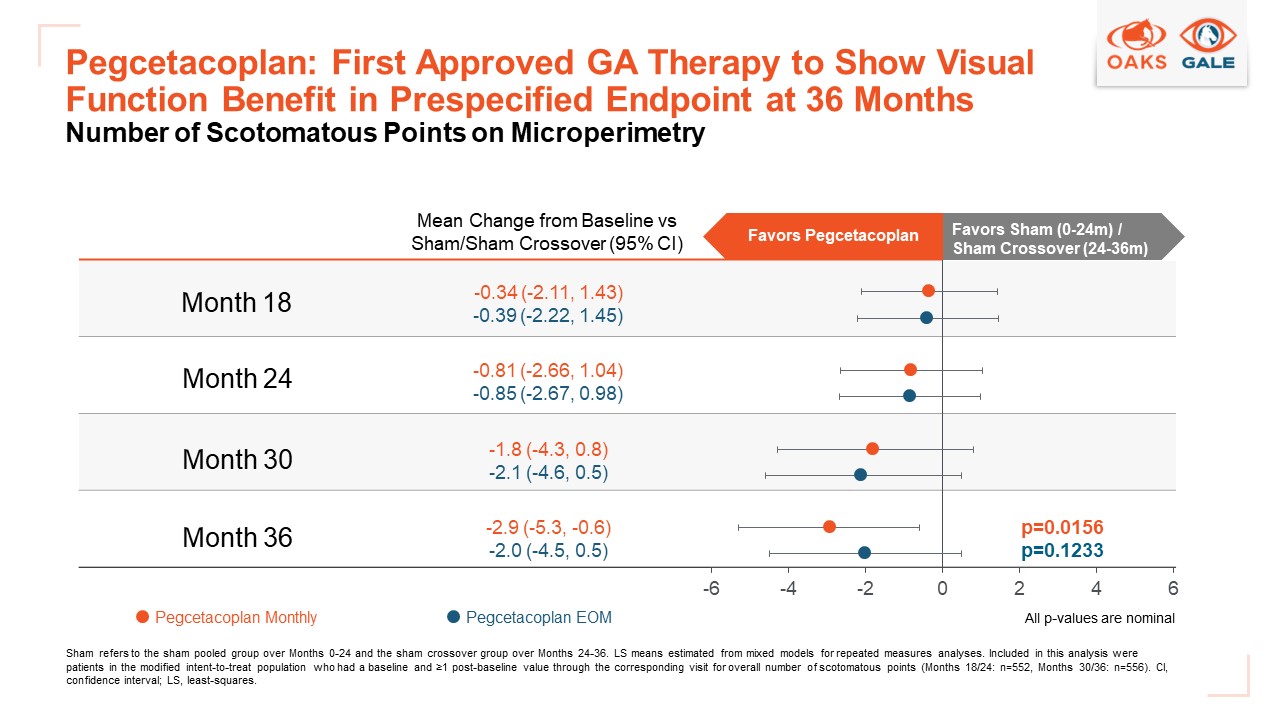
The findings were presented at the Clinical Trials at the Summit Meeting. SYFOVRE is the only approved GA treatment to show a visual function benefit in a prespecified endpoint, reducing the development of new scotomatous points compared to a sham crossover group.
The GALE study, consisting of 792 participants, evaluates the long-term efficacy and safety of SYFOVRE. The Phase 3 OAKS and DERBY studies supporting these results showed that both monthly and every-other-month doses reduced GA lesion growth and maintained a favorable safety profile.
However, SYFOVRE is associated with risks such as endophthalmitis, retinal detachments, and increased intraocular pressure.
Positive
- SYFOVRE preserved visual function for 36 months in GA patients.
- SYFOVRE is the only approved treatment to show visual function benefit in a prespecified endpoint for GA.
- The GALE study had strong participation with 792 patients.
- Both monthly and every-other-month dosing showed reduced GA lesion growth in Phase 3 studies.
- Positive safety profile maintained in long-term studies.
Negative
- SYFOVRE presents risks of endophthalmitis and retinal detachments.
- Use of SYFOVRE is associated with increased rates of neovascular AMD or choroidal neovascularization.
- SYFOVRE treatment linked to intraocular inflammation and increased intraocular pressure.
- Patients may need to be monitored closely for signs of severe adverse effects.
News Market Reaction 1 Alert
On the day this news was published, APLS gained 3.29%, reflecting a moderate positive market reaction.
Data tracked by StockTitan Argus on the day of publication.
- SYFOVRE is the only approved GA treatment to demonstrate a visual function benefit in a prespecified endpoint
- Data presented at the Clinical Trials at the Summit Meeting
WALTHAM, Mass., June 10, 2024 (GLOBE NEWSWIRE) -- Apellis Pharmaceuticals, Inc. (Nasdaq: APLS) today announced that SYFOVRE® (pegcetacoplan injection) preserved visual function at 36 months in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). These positive data from the GALE long-term extension study were presented at the Clinical Trials at the Summit (CTS) Meeting on June 8 in Park City, Utah.
“SYFOVRE is the only approved GA treatment to show a benefit on visual function in a prespecified endpoint,” said Dilsher Dhoot, M.D., presenting author, vitreoretinal surgeon, California Retina Consultants, Santa Barbara, CA. “The vision loss caused by GA is devastating for patients, taking away their ability to drive and read. These groundbreaking data clearly demonstrate SYFOVRE’s potential to make a meaningful difference for patients.”
In a prespecified microperimetry endpoint, patients developed fewer new scotomatous points with 36 months of both continuous monthly (p=0.0156) and every-other-month (p=0.1233) treatment compared to patients from the sham crossover group (all p-values nominal). Scotomatous points measure areas of the retina that have lost all light sensitivity and therefore are no longer functioning.
“These results further reinforce the importance of slowing GA lesion growth to preserve visual function, adding to the largest body of evidence for a GA treatment,” said Caroline Baumal, M.D., chief medical officer, Apellis. “As leaders in GA, we are committed to advancing our understanding of the benefits of SYFOVRE on this progressive and long-term disease.”
View full table by clicking on this link or the image below:
About the GALE Long-Term Extension Study
GALE (n=792) is a Phase 3, multicenter, open-label, extension study to evaluate the long-term efficacy and safety of SYFOVRE® (pegcetacoplan injection) in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The objectives of the study are to evaluate the long-term incidence and severity of ocular and systemic treatment emergent adverse events as well as change in the total area of GA lesions as measured by fundus autofluorescence. More than 80-percent of participants who completed the OAKS and DERBY studies entered the GALE study. GALE also includes 10 patients who were previously enrolled in the Phase 1b study of pegcetacoplan for GA.
Patients in the sham crossover group completed sham treatment from Months 0-24 in the Phase 3 OAKS study and received SYFOVRE from Months 24-36. Microperimetry was a key secondary endpoint measured only in the OAKS study, and therefore, patients who crossed over from the OAKS study were included in this analysis.
About the Phase 3 OAKS and DERBY Studies
OAKS (n=637) and DERBY (n=621) are Phase 3, multicenter, randomized, double-masked, sham-controlled studies comparing the efficacy and safety of SYFOVRE® (pegcetacoplan injection) with sham injections across a broad and heterogenous population of patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The studies evaluated the efficacy of monthly and every-other-month SYFOVRE in patients with GA assessed by change in the total area of GA lesions from baseline as measured by fundus autofluorescence.
In Phase 3 studies at 24 months, both every-other-month and monthly SYFOVRE reduced GA lesion growth with increasing effects over time and showed a well-demonstrated safety profile.
About SYFOVRE® (pegcetacoplan injection)
SYFOVRE® (pegcetacoplan injection) is the first-ever approved therapy for geographic atrophy (GA). By targeting C3, SYFOVRE is designed to provide comprehensive control of the complement cascade, part of the body’s immune system. SYFOVRE is approved in the United States for the treatment of GA secondary to age-related macular degeneration.
About Geographic Atrophy (GA)
Geographic atrophy (GA) is an advanced form of age-related macular degeneration and a leading cause of blindness worldwide, impacting more than one million Americans and five million people worldwide.1,2 It is a progressive and irreversible disease caused by the growth of lesions, which destroy the retinal cells responsible for vision. The vision loss caused by GA severely impairs independence and quality of life by making it difficult to participate in daily activities. On average, it takes only 2.5 years for GA lesions to start impacting the fovea, which is responsible for central vision.3
U.S. Important Safety Information for SYFOVRE® (pegcetacoplan injection)
CONTRAINDICATIONS
- SYFOVRE is contraindicated in patients with ocular or periocular infections, and in patients with active intraocular inflammation
WARNINGS AND PRECAUTIONS
- Endophthalmitis and Retinal Detachments
- Intravitreal injections, including those with SYFOVRE, may be associated with endophthalmitis and retinal detachments. Proper aseptic injection technique must always be used when administering SYFOVRE to minimize the risk of endophthalmitis. Patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment without delay and should be managed appropriately.
- Retinal Vasculitis and/or Retinal Vascular Occlusion
- Retinal vasculitis and/or retinal vascular occlusion, typically in the presence of intraocular inflammation, have been reported with the use of SYFOVRE. Cases may occur with the first dose of SYFOVRE and may result in severe vision loss. Discontinue treatment with SYFOVRE in patients who develop these events. Patients should be instructed to report any change in vision without delay.
- Neovascular AMD
- In clinical trials, use of SYFOVRE was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
12% when administered monthly,7% when administered every other month and3% in the control group) by Month 24. Patients receiving SYFOVRE should be monitored for signs of neovascular AMD. In case anti-Vascular Endothelial Growth Factor (anti-VEGF) is required, it should be given separately from SYFOVRE administration.
- In clinical trials, use of SYFOVRE was associated with increased rates of neovascular (wet) AMD or choroidal neovascularization (
- Intraocular Inflammation
- In clinical trials, use of SYFOVRE was associated with episodes of intraocular inflammation including: vitritis, vitreal cells, iridocyclitis, uveitis, anterior chamber cells, iritis, and anterior chamber flare. After inflammation resolves, patients may resume treatment with SYFOVRE.
- Increased Intraocular Pressure
- Acute increase in IOP may occur within minutes of any intravitreal injection, including with SYFOVRE. Perfusion of the optic nerve head should be monitored following the injection and managed as needed.
ADVERSE REACTIONS
- Most common adverse reactions (incidence ≥
5% ) are ocular discomfort, neovascular age-related macular degeneration, vitreous floaters, conjunctival hemorrhage.
Please see accompanying full Prescribing Information for more information.
About Apellis
Apellis Pharmaceuticals, Inc. is a global biopharmaceutical company that combines courageous science and compassion to develop life-changing therapies for some of the most challenging diseases patients face. We ushered in the first new class of complement medicine in 15 years and now have two approved medicines targeting C3. These include the first-ever therapy for geographic atrophy, a leading cause of blindness around the world. We believe we have only begun to unlock the potential of targeting C3 across serious retinal, rare, and neurological diseases. For more information, please visit http://apellis.com or follow us on Twitter and LinkedIn.
Apellis Forward-Looking Statement
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors and other factors discussed in the “Risk Factors” section of Apellis’ Annual Report on Form 10-K with the Securities and Exchange Commission on February 27, 2024 and the risks described in other filings that Apellis may make with the Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof, and Apellis specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
Media Contact:
Lissa Pavluk
media@apellis.com
617.977.6764
Investor Contact:
Meredith Kaya
meredith.kaya@apellis.com
617.599.8178
1Rudnicka AR, Jarrar Z, Wormald R, et al. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta analysis. Ophthalmology 2012;119:571–580.
2Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–116.
3Lindblad AS, et al, and AREDS Research Group. Arch Ophthalmol. 2009;127(9):1168-1174.