Akebia Presents Results from its PRO2TECT Global Phase 3 Program of Vadadustat for the Treatment of Anemia due to Chronic Kidney Disease in Adult Patients Not on Dialysis During Late-Breaking Session at American Society of Nephrology Kidney Week 2020 Reima
Rhea-AI Summary
On October 23, 2020, Akebia Therapeutics (Nasdaq: AKBA) presented clinical data from its PRO2TECT Phase 3 program at ASN Kidney Week. The program assessed the efficacy and safety of vadadustat versus darbepoetin alfa for treating anemia due to chronic kidney disease (CKD) in patients not on dialysis. Vadadustat met its primary and key secondary efficacy endpoints, achieving non-inferiority. However, it failed to meet the primary safety endpoint concerning major adverse cardiovascular events (MACE), although further analysis indicated no significant increase in cardiovascular risk for U.S. patients.
Positive
- Vadadustat achieved primary and key secondary efficacy endpoints in the PRO2TECT studies.
- Demonstrated non-inferiority to darbepoetin alfa based on mean hemoglobin change.
- No clinically meaningful increase in cardiovascular risk in U.S. patients treated within guidelines.
Negative
- Vadadustat did not meet the primary safety endpoint regarding MACE.
- Increased risk of adverse events compared to darbepoetin alfa, particularly concerning hypertension and end-stage renal disease.
News Market Reaction 1 Alert
On the day this news was published, AKBA declined 3.05%, reflecting a moderate negative market reaction.
Data tracked by StockTitan Argus on the day of publication.
CAMBRIDGE, Mass., Oct. 23, 2020 /PRNewswire/ -- Akebia Therapeutics, Inc. (Nasdaq: AKBA), today announced the presentation of clinical data from its PRO2TECT global Phase 3 program that evaluated the efficacy and safety of vadadustat, Akebia's investigational oral hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), versus darbepoetin alfa for the treatment of anemia due to chronic kidney disease (CKD) in adult patients not on dialysis, at American Society of Nephrology Kidney Week 2020 Reimagined (ASN Kidney Week). As previously announced, vadadustat achieved the primary and key secondary efficacy endpoints in each of the two studies in the program, but did not meet the primary safety endpoint. Newly presented pre-specified regional analyses of the PRO2TECT program show vadadustat demonstrated no clinically meaningful increase in cardiovascular risk compared to darbepoetin alfa in analyses of MACE, expanded MACE and all-cause mortality in U.S. patients treated to a target hemoglobin (Hb) range of 10 to 11 g/dL, consistent with U.S. treatment guidelines.
"The PRO2TECT results show that orally administered vadadustat achieved both primary and secondary efficacy endpoints in patients not on dialysis with anemia associated with CKD. The newly presented analyses showed that there were regional differences with respect to MACE, expanded MACE and all-cause mortality, consistent with well-known, differing regional hemoglobin treatment target guidelines," said Glenn Chertow, M.D., M.P.H., Professor of Medicine, Chief, Division of Nephrology at Stanford University, and Co-Chair of the independent Executive Steering Committee for PRO2TECT and INNO2VATE. "The analyses also confirm there was no increased cardiovascular risk associated with vadadustat across the U.S. patients treated to a target hemoglobin range of 10 to 11 g/dL."
The PRO2TECT data are being presented today during a late-breaking oral presentation, titled "Global Phase 3 Clinical Trials of Vadadustat vs Darbepoetin Alfa for Treatment of Anemia in Patients with Non-Dialysis-Dependent Chronic Kidney Disease" (Abstract FR-OR54) at ASN Kidney Week. Akebia's vadadustat development program also includes INNO2VATE, the global Phase 3 program for the treatment of anemia due to CKD in adult patients on dialysis. Results from this program were presented at ASN Kidney Week in an oral presentation on October 22, 2020.
Highlights of the PRO2TECT ASN Kidney Week Presentation:
Efficacy:
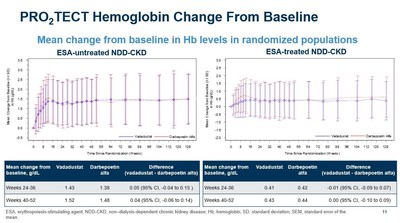
- As previously reported, vadadustat achieved the primary and key secondary efficacy endpoints in each of the two PRO2TECT studies, which demonstrated non-inferiority of vadadustat to darbepoetin alfa as measured by a mean change in Hb between baseline and the primary evaluation period (weeks 24 to 36) and secondary evaluation period (weeks 40 to 52). Non-inferiority was achieved as the lower bound of the
95% confidence interval for the between-group difference of the mean Hb change did not fall below the pre-specified non-inferiority margin (-0.75 g/dL).
Safety:
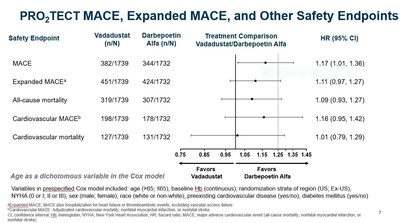
- As previously reported, vadadustat did not meet the primary safety endpoint of the PRO2TECT program defined as non-inferiority of vadadustat versus darbepoetin alfa in time to first occurrence of major adverse cardiovascular events (MACE), which is the composite of all-cause mortality, non-fatal myocardial infarction (MI), or non-fatal stroke across both PRO2TECT studies.
- The analysis of the primary safety endpoint used a Cox regression model that was pre-specified to be adjusted for certain variables, including age dichotomized >65 and ≤65 years.
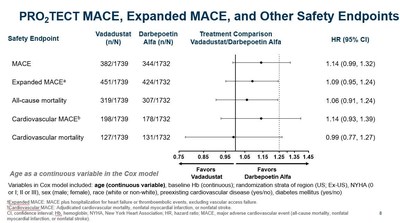
- In order to optimize the Cox model used to analyze the primary safety endpoint and increase its statistical power in regional subsets, age was rescaled from dichotomous to a continuous variable.
- In the optimized Cox model, the hazard ratios (HR) were attenuated and the confidence intervals (CI) were narrowed.
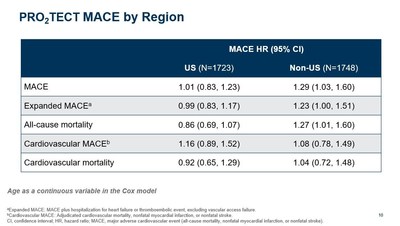
- The PRO2TECT study analysis plan was prospectively designed to analyze the effect of regional differences, most notably, well-known differences in Hb treatment targets. Within the study, U.S. patients were treated to a target Hb range of 10 to 11 g/dL and non-U.S. patients were treated to a target Hb range of 10 to 12 g/dL.
- In a pre-specified regional analysis using age as a dichotomous variable, vadadustat was not associated with a clinically meaningful increase in cardiovascular risk compared to darbepoetin alfa in U.S. patients treated to a target Hb range of 10 to 11 g/dL, with a MACE HR of 1.06 (0.87, 1.29). This was confirmed using age as a continuous variable as shown below.
- The incidence of treatment emergent adverse events during the ESA-untreated patients (Correction) study in the vadadustat-treated patients was
90.9% , and91.6% in darbepoetin alfa-treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa-treated patients were end-stage renal disease (34.7% /35.2% ), hypertension (17.7% / 22.1.% ), hyperkalemia (12.3.% /15.6% ), urinary tract infection (12.9% /12.0% ), diarrhea (13.9% /10.0% ), peripheral oedema (12.5% /10.5% ), fall (9.6% /10% ) and nausea (10% /8.2% ). Serious treatment emergent adverse events were65.3% for vadadustat-treated patients and64.5% for darbepoetin alfa-treated patients. The incidence of treatment emergent adverse events during the ESA-treated patients (Conversion) study in vadadustat treated patients was89.1% and87.7% in darbepoetin alfa-treated patients. During the study, the most common treatment emergent adverse events reported in vadadustat/darbepoetin alfa-treated patients were end-stage renal disease (27.5% /28.4% ), hypertension (14.4% /14.8% ), urinary tract infection (12.2% /14.5% ), diarrhea (13.8.% / 8.8.% ), peripheral oedema (9.9% /10.1% ) and pneumonia (10.0% /9.7% ). Serious treatment emergent adverse events were58.5% for vadadustat-treated patients and56.6% for darbepoetin alfa-treated patients.
"The newly presented pre-specified analyses of PRO2TECT showed that vadadustat had no clinically meaningful increase in cardiovascular risk in U.S. patients not on dialysis treated to a target hemoglobin range of 10 to 11 g/dL, consistent with U.S. treatment guidelines. Upon submission of our NDA for vadadustat, we expect that the potential approval of vadadustat as a treatment for patients not on dialysis will be a review issue for the FDA, and we continue to believe that these important analyses, together with the totality of data from our Phase 3 program, will inform that review," said John P. Butler, President and CEO of Akebia Therapeutics. "We remain confident that vadadustat has a straightforward path to potential approval for the treatment of patients on dialysis, globally."
Akebia plans to submit to the U.S. Food and Drug Administration (FDA) a New Drug Application (NDA) for vadadustat for the treatment of anemia due to CKD in adult dialysis-dependent and non-dialysis dependent patients as early as possible in 2021. In addition, Akebia and its collaborator, Otsuka Pharmaceutical Co. Ltd., are working together to further analyze these study results to support a Marketing Authorization Application (MAA) for submission to the European Medicines Agency (EMA) next year.
Investor Briefing Webcast
Akebia management will host an investor briefing webcast with Dr. Glenn Chertow on October 23, 2020 at 4:10 p.m. ET to review highlights of the global Phase 3 vadadustat data presentations from ASN Kidney Week. To access Akebia's investor briefing webcast and the accompanying slides please log into the Investors section of the Company's website at https://ir.akebia.com and proceed to the events and presentations page at https://ir.akebia.com/events-and-presentations. Please connect to the Company's website at least 10 minutes prior to the online event to ensure adequate time for any software download that may be required to view the webcast. After the webcast concludes, a replay of the event will be available at that same location until October 29, 2020
About the PRO2TECT Global Phase 3 Program of Vadadustat
Akebia's global PRO2TECT program included two separate Phase 3 studies of ESA-untreated patients (Correction) and ESA-treated patients (Conversion), which collectively enrolled 3,476 adult patients not on dialysis with anemia due to CKD. Both PRO2TECT studies were global, multicenter, open label (sponsor blind), active-controlled (darbepoetin alfa - an injectable erythropoiesis stimulating agent (ESA)), non-inferiority studies. In both studies, patients were randomized 1:1 to receive either vadadustat or darbepoetin alfa. Efficacy and safety results were measured against non-inferiority margins agreed upon with the FDA and the EMA.
About Anemia due to Chronic Kidney Disease (CKD)
Anemia is a condition in which a person lacks enough healthy red blood cells to carry adequate oxygen to the body's tissues. It commonly occurs in people with CKD because their kidneys do not produce enough erythropoietin (EPO), a hormone that helps regulate production of red blood cells. Anemia due to CKD can have a profound impact on a person's quality of life as it can cause fatigue, dizziness, shortness of breath and cognitive dysfunction. Left untreated, anemia leads to deterioration in health and is associated with increased morbidity and mortality in people with CKD.
About Vadadustat
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor designed to mimic the physiologic effect of altitude on oxygen availability. At higher altitudes, the body responds to lower oxygen availability with stabilization of hypoxia-inducible factor, which can lead to increased red blood cell production and improved oxygen delivery to tissues. Vadadustat is in global Phase 3 development for the treatment of anemia due to CKD and is not approved by the U.S. Food and Drug Administration (FDA) or any regulatory authority with the exception of Japan's Ministry of Health, Labour and Welfare (MHLW). In Japan, vadadustat is approved as a treatment for anemia due to CKD in both dialysis-dependent and non-dialysis dependent adult patients.
About Akebia Therapeutics
Akebia Therapeutics, Inc. is a fully integrated biopharmaceutical company with the purpose to better the lives of people impacted by kidney disease. The Company was founded in 2007 and is headquartered in Cambridge, Massachusetts. For more information, please visit our website at www.akebia.com, which does not form a part of this release.
Forward Looking Statements
Statements in this press release regarding Akebia's strategy, plans, prospects, expectations, beliefs, intentions and goals are forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, including but not limited to statements regarding the expectation that the potential approval of vadadustat in the U.S. as a treatment for patients not on dialysis will be a review issue for the FDA; the belief that newly presented analyses of PRO2TECT and the totality of the data from the global Phase 3 program of vadadustat will inform the FDA's review of treatment of patients not on dialysis with vadadustat; the potential for obtaining approval of vadadustat in dialysis; the potential indications for and benefits of vadadustat; submitting filings for marketing approval of vadadustat, and the timing thereof; and the clinical opportunity for vadadustat. The terms "believe," "confident," "expect," "plan," "potential," "will," "working" and similar references are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement, including the timing and content of advice given and decisions made by health authorities, including approval and labeling decisions; the actual time it takes to make regulatory submissions for vadadustat to health authorities, including the submission of the NDA to the FDA and the submission of the MAA to the EMA; risks associated with the Priority Review Voucher for vadadustat; the potential direct or indirect impact of the COVID-19 pandemic on our business, operations, and the markets and communities in which we and our partners, collaborators, vendors and customers operate; manufacturing and quality risks; risks associated with management and key personnel changes and transitional periods; the actual funding required to continue to commercialize our commercial product, to develop and commercialize vadadustat, and to operate the Company; market acceptance and coverage and reimbursement of our commercial product and vadadustat, if approved; the risks associated with potential generic entrants for our commercial product and vadadustat, if approved; early termination of any of Akebia's collaborations; Akebia's and its collaborators' ability to satisfy their obligations under Akebia's collaboration agreements; the competitive landscape for our commercial product and vadadustat; the scope, timing, and outcome of any legal, regulatory and administrative proceedings; changes in the economic and financial conditions of the businesses of Akebia and its collaborations partners and vendors; and Akebia's ability to obtain, maintain and enforce patent and other intellectual property protection for our commercial product, vadadustat and any other product candidates. Other risks and uncertainties include those identified under the heading "Risk Factors" in Akebia's Quarterly Report on Form 10-Q for the quarter ended June 30, 2020 and other filings that Akebia may make with the U.S. Securities and Exchange Commission in the future. These forward-looking statements (except as otherwise noted) speak only as of the date of this press release, and Akebia does not undertake, and specifically disclaims, any obligation to update any forward-looking statements contained in this press release.
Investor Contact
Kristen K. Sheppard, Esq.
Ir@akebia.com
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-pro2tect-global-phase-3-program-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-not-on-dialysis-during-late-breaking-session-at-american-society-of-nephrol-301158891.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/akebia-presents-results-from-its-pro2tect-global-phase-3-program-of-vadadustat-for-the-treatment-of-anemia-due-to-chronic-kidney-disease-in-adult-patients-not-on-dialysis-during-late-breaking-session-at-american-society-of-nephrol-301158891.html
SOURCE Akebia Therapeutics