Restore EF Study Shows Heart Function, Symptom Improvements for High-Risk PCI Patients Supported by Impella
Consistent evidence supports benefits of complete revascularization on LVEF, heart failure symptoms
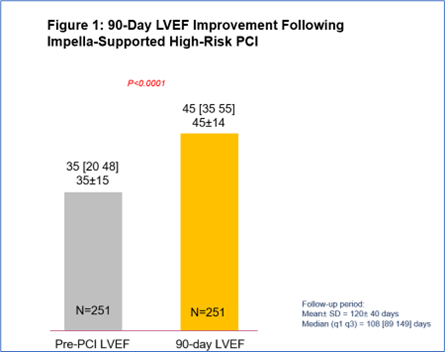
Figure 1: 90-Day LVEF Improvement Following Impella-Supported High-Risk PCI
Restore EF is a prospective, multi-center study evaluating the best practices in contemporary PCI practice, including more complete revascularization. Participants received an Impella-supported high-risk PCI, also called an “on-pump PCI,” at one of 22 sites across
-
A
29% relative improvement from baseline LVEF (n=251, p<0.0001), with a significantly greater improvement in LVEF for those who had a complete revascularization (characterized by a residual SYNTAX score of 0). (See figures 1 and 2) -
Significant improvement in heart failure symptoms, with an overall
76% reduction in New York Heart Association Class III or IV heart failure symptoms (n=274, p<0.001). (See figure 3) -
Significant improvement in angina symptoms, with an overall
97% reduction in Canadian Cardiovascular Society Class III or IV angina symptoms (n=260, p<0.0001). (See figure 4)
Participants with higher baseline LVEF (greater than
“The Restore EF study results add to the growing body of evidence demonstrating that Impella-supported high-risk PCI can lead to a more complete revascularization and considerable LVEF improvement,” said
An accompanying editorial published in JSCAI, written by physicians from
Results from Restore EF and the PROTECT III study, which published in the
“These results further demonstrate that high-risk PCI procedures supported by Impella employing contemporary best practices are safe and effective, providing a treatment option for patients who have historically had limited options to improve their quality of life,” said
Restore EF and PROTECT III are the latest in a growing list of studies that demonstrate Impella-supported high-risk PCI leads to improvement in LVEF:
-
Journal of the American College of Cardiology , 2009 – The PROTECT I trial found patients who had a Protected PCI with Impella had a31% improvement in LVEF at 30-day follow up. (From 26 ±6% to 34 ±11% , p=0.003). -
Catheterization and Cardiovascular Interventions, 2011 – This study, led by Maini, et al., found a
17% improvement in LVEF at follow up, after a Protected PCI with Impella (p<0.0001). -
Circulation, 2012 – The PROTECT II randomized controlled trial found Protected PCI with Impella led to a
58% improvement in NYHA Class III and IV heart failure symptoms at 90 days (p<0.001). The trial also found, during follow up after Protected PCI with Impella, patients had a22% improvement in LVEF (p<0.001). -
Journal of Interventional Cardiology , 2013 – This study, led by O’Neill, et al., suggests that early initiation of hemodynamic support prior to PCI with Impella 2.5 is associated with more complete revascularization and improved survival to discharge compared to post-PCI support (65.1% vs.40.7% , p<0.003). -
American Journal of Cardiology , 2013 – An analysis of the PROTECT II randomized controlled trial by Dangas, et al., found Impella use led to a29% reduction in major adverse cardiac and cerebrovascular events (MACCE) at 90 days, compared to the use of the intra-aortic balloon pump (IABP) (p=0.042). -
Journal of Interventional Cardiology , 2019 – This study, led by Burzotta, et al., found six months after a Protected PCI, the percentage of patients with LVEF greater than or equal to35% increased by205% , from22% to67% (n=79, p≤0.001). The study also found more complete revascularization was associated with significant LVEF improvement and survival.
These studies have led to the PROTECT IV randomized controlled trial, which began enrolling patients in
Additional information about the Restore EF study, including case studies and interviews with the study’s authors, is available on HeartRecovery.com.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® with SmartAssist® are
ABOUT
Based in
FORWARD-LOOKING STATEMENTS
Any forward-looking statements are subject to risks and uncertainties such as those described in
View source version on businesswire.com: https://www.businesswire.com/news/home/20220816005340/en/
Media Contact:
Associate Director,
+1 (978) 882-8491
jleary@abiomed.com
Investor Contact:
Vice President and Chief Financial Officer
+1 (978) 646-1680
ttrapp@abiomed.com
Source:







