XPhyto Reports Excellent Rotigotine In-Vitro/Ex-Vivo Results for Parkinson's Disease Treatment
XPhyto Therapeutics Corp. (CSE:XPHY, OTCQB:XPHYF) announced positive results from its human skin cadaver study of the Rotigotine transdermal patch, showing absorption profiles similar to a leading brand. The study confirms that XPhyto's new formulation is ready for clinical evaluation, marking a significant step toward commercialization. Rotigotine, used for treating Parkinson's disease, reported a market value of $518 million in 2021, expected to rise to $766 million by 2030. XPhyto's transdermal technology represents a scalable opportunity within the $20 billion global patch market.
- None.
- None.
Insights
Analyzing...
VANCOUVER, BC and UTTENWEILER, GERMANY / ACCESSWIRE / October 18, 2022 / XPhyto Therapeutics Corp. (CSE:XPHY)(OTCQB:XPHYF)( FSE:4XT) ("XPhyto" or the "Company") is pleased to report the results of its Rotigotine transdermal ("TDS") patch human skin cadaver study and dissolution data. The Company's Rotigotine patch is based on the TDS platform technology developed by its wholly owned German subsidiary, Vektor Pharma TF GmbH ("Vektor").
Further to the Company's product update on October 11, 2022, XPhyto is pleased to report excellent results from its recent Rotigotine TDS human cadaver skin absorption study. The study compared drug absorption between XPhyto's optimized new formula and the name brand product in three separate samples over a 24-hour period in accordance with EMA's Guideline on quality of transdermal patches.
The study results demonstrate exceptionally similar dissolution and absorption profiles between XPhyto's drug formulation and the name brand product ("NB"):
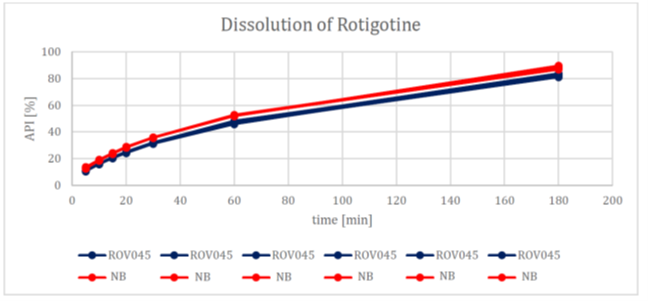
Figure 1. XPhyto results demonstrating dissolution data in accordance with USP acceptance criteria for its Rotigotine TDS formulation (ROV045) compared to the name brand product (NB).
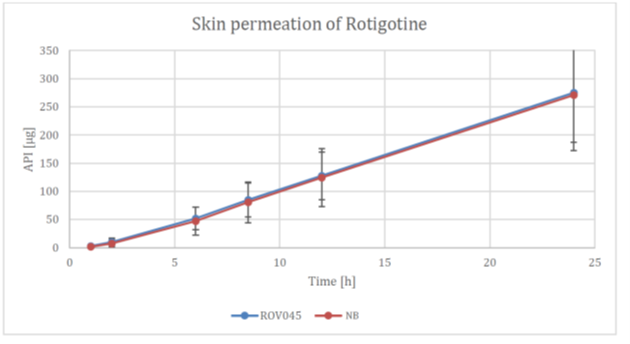
Figure 2. XPhyto results demonstrating total average API absorption over 24 hours for its Rotigotine TDS formulation (ROV045) compared to the name brand product (NB).
"As our lead product we are extremely pleased with these results. They have exceeded our expectations," said Hugh Rogers, XPhyto CEO & Director. "We are confident that our Rotigotine patch is fully optimized and ready for further human clinical evaluation. This is a major milestone in the pathway to product commercialization."
Rotigotine is a non-ergoline dopamine agonist approved for the treatment of Parkinson's disease and restless legs syndrome (RLS) in Europe and the United States. The active pharmaceutical ingredient is not well absorbed via oral delivery and is formulated as a once-daily TDS patch to increase bioavailability and provide a slow and steady supply of the drug over the course of 24 hours. The therapeutic market for Parkinson's disease is over 10 million people worldwide and growing. The top selling name brand product launched by the originator in 2007 independently sold over
XPhyto's Rotigotine transdermal product is a single product based on its
Vektor, a wholly owned XPhyto subsidiary, is a German narcotics manufacturer, developer, and researcher located in the district of Biberach, Baden-Württemberg, Germany. For over a decade, the company and its team have been leaders in the design, testing and manufacture of innovative, non-invasive drug delivery systems, particularly transdermal patches and sub-lingual strips for the delivery of active pharmaceutical ingredients for the treatment of pain and neurological conditions. According to Precedence Research, the global drug delivery market was valued at US
About XPhyto Therapeutics Corp.
XPhyto Therapeutics Corp. is a diversified bioscience accelerator focused on next-generation drug formulation, diagnostic, and new active pharmaceutical ingredient investment opportunities, including: precision transdermal and oral dissolvable drug formulations; rapid, low-cost infectious disease and oral health screening tests; and manufacture, standardization, and evaluation of psychedelic compounds for the treatment of neurological conditions. The Company has research and development operations in North America and Europe, with an operational focus in Germany, and is currently focused on regulatory approval and commercialization of medical products for European markets.
XPhyto Therapeutics Corp.
Hugh Rogers, CEO and Director
Investor inquiries:
T: 780-818-6422
E: info@xphyto.com
Forward looking statements
This news release includes statements containing forward-looking information within the meaning of applicable Canadian securities law ("forward-looking statements"). Forward-looking statements are frequently characterized by words such as "develop", "plan", "continue", "expect", "project", "intend", "believe", "anticipate", "estimate", "potential", "propose" and other similar words, or statements that certain events or conditions "may" or "will" occur, and in this release include the statement regarding the Company's goal of building a successful diagnostic, drug delivery, and medical cannabis company. Forward-looking statements are only predictions based on the opinions and estimates of management at the date the statements are made and are subject to a variety of risks and uncertainties and other factors that could cause actual events or results to differ materially from those projected in the forward-looking statements, including: that the Company may not succeed in developing a commercial product; that the sale of products may not be a viable business; that the Company may be unable to scale its business; product liability risks; product regulatory risk; general economic conditions; adverse industry events; future legislative and regulatory developments; inability to access sufficient capital from internal and external sources, and/or inability to access sufficient capital on favourable terms; currency risks; competition; international risks; and other risks beyond the Company's control. The Company is under no obligation, and expressly disclaims any intention or obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as expressly required by applicable law. Neither the CSE nor its Market Regulator (as that term is defined in the policies of the CSE) accepts responsibility for the adequacy or accuracy of this news release.
SOURCE: XPhyto Therapeutics Corp.
View source version on accesswire.com:
https://www.accesswire.com/720788/XPhyto-Reports-Excellent-Rotigotine-In-VitroEx-Vivo-Results-for-Parkinsons-Disease-Treatment







