Vera Therapeutics Announces 96-week eGFR Stabilization in ORIGIN Phase 2b Study of Atacicept in IgAN in a Late-Breaking Oral Presentation at the American Society of Nephrology Kidney Week 2024
Vera Therapeutics (NASDAQ: VERA) announced 96-week data from its ORIGIN Phase 2b trial of atacicept in IgA nephropathy (IgAN), showing stabilized kidney function. The results, presented at ASN Kidney Week 2024, demonstrated -66% reduction in Gd-IgA1, 75% resolution of hematuria, -52% reduction in proteinuria, and a mean annualized eGFR slope of -0.6 mL/min/1.73m2/year. The trial maintained a 90% completion rate with a favorable safety profile. The company expects topline results from Phase 3 ORIGIN 3 trial in Q2 2025, with planned BLA submission later that year.
Vera Therapeutics (NASDAQ: VERA) ha annunciato i dati a 96 settimane del suo trial di Fase 2b ORIGIN riguardante l'atacicept per la nefropatia da IgA (IgAN), mostrando una funzione renale stabilizzata. I risultati, presentati alla ASN Kidney Week 2024, hanno dimostrato una riduzione del -66% di Gd-IgA1, una risoluzione del 75% dell'ematuria, una riduzione del -52% della proteinuria e una pendenza media annualizzata di eGFR di -0,6 mL/min/1.73m2/anno. Il trial ha mantenuto una percentuale di completamento del 90% con un profilo di sicurezza favorevole. L'azienda prevede risultati iniziali dal trial di Fase 3 ORIGIN 3 nel secondo trimestre del 2025, con una prevista presentazione della BLA entro la fine dell'anno.
Vera Therapeutics (NASDAQ: VERA) anunció datos a 96 semanas de su ensayo de Fase 2b ORIGIN sobre atacicept en nefropatía por IgA (IgAN), mostrando una función renal estabilizada. Los resultados, presentados en la ASN Kidney Week 2024, demostraron una reducción del -66% en Gd-IgA1, una resolución del 75% de la hematuria, una reducción del -52% en proteinuria y una pendiente de eGFR anualizada media de -0,6 mL/min/1.73m2/año. El ensayo mantuvo una tasa de finalización del 90% con un perfil de seguridad favorable. La empresa espera resultados preliminares del ensayo de Fase 3 ORIGIN 3 en el segundo trimestre de 2025, con una presentación de BLA programada para más tarde ese año.
베라 테라퓨틱스 (NASDAQ: VERA)는 IgA 신병증 (IgAN) 치료를 위한 atacicept의 ORIGIN 2b 임상 시험에서 96주 데이터를 발표하며, 안정화된 신장 기능을 보여주었습니다. ASN Kidney Week 2024에서 발표된 결과는 Gd-IgA1의 -66% 감소, 혈뇨의 75% 해결, 단백뇨의 -52% 감소, 평균 연간 eGFR 기울기가 -0.6 mL/min/1.73m2/년임을 나타냈습니다. 시험은 90%의 완료율과 우호적인 안전성 프로파일을 유지했습니다. 회사는 2025년 2분기에 ORIGIN 3 3상 시험의 주요 결과를 기대하고 있으며, 그 해 후반에 BLA 제출을 계획하고 있습니다.
Vera Therapeutics (NASDAQ: VERA) a annoncé des données de 96 semaines de son essai de Phase 2b ORIGIN concernant l'atacicept dans la néphropathie à IgA (IgAN), montrant une fonction rénale stabilisée. Les résultats, présentés lors de l'ASN Kidney Week 2024, ont démontré une réduction de -66% du Gd-IgA1, une résolution de 75% de l'hématurie, une réduction de -52% de la protéinurie et une pente annualisée moyenne de eGFR de -0,6 mL/min/1.73m2/an. L'essai a maintenu un taux d'achèvement de 90% avec un profil de sécurité favorable. La société s'attend à des résultats préliminaires de l'essai de Phase 3 ORIGIN 3 au deuxième trimestre 2025, avec une soumission de BLA prévue plus tard cette année.
Vera Therapeutics (NASDAQ: VERA) hat die 96-Wochen-Daten seiner ORIGIN-Phase-2b-Studie zu Atacicept bei IgA-Nephropathie (IgAN) veröffentlicht, die eine stabilisierte Nierenfunktion zeigen. Die Ergebnisse, die auf der ASN Kidney Week 2024 präsentiert wurden, zeigten eine Reduktion von -66% in Gd-IgA1, eine 75%ige Lösung der Hämaturie, eine Reduktion von -52% in der Proteinurie und eine durchschnittliche jährliche eGFR-Schräge von -0,6 mL/min/1.73m2/Jahr. Die Studie erzielte eine Abschlussquote von 90% mit einem günstigen Sicherheitsprofil. Das Unternehmen erwartet die erste Ergebnisbekanntgabe der Phase-3-Studie ORIGIN 3 im 2. Quartal 2025, mit einer geplanten BLA-Einreichung später im Jahr.
- 96-week data showed significant disease improvements with -66% Gd-IgA1 reduction
- High treatment completion rate of 90% with favorable safety profile
- 75% of participants achieved hematuria resolution
- 52% reduction in proteinuria
- Stabilized kidney function with minimal eGFR decline (-0.6 mL/min/1.73m2/year)
- None.
Insights
The 96-week ORIGIN Phase 2b data for atacicept demonstrates remarkable disease-modifying potential in IgAN patients. Key findings include
The
- Long-term improvements observed in the quartet of findings defining disease modification supports atacicept’s potential to prevent kidney failure in patients with IgAN;
- Long-term results from the ORIGIN Phase 2b study were simultaneously published in the Journal of the American Society of Nephrology;
- Company will host an investor call and webcast on Monday October 28 at 8:00 AM ET
BRISBANE, Calif., Oct. 26, 2024 (GLOBE NEWSWIRE) -- Vera Therapeutics, Inc. (Nasdaq: VERA), a late clinical-stage biotechnology company focused on developing and commercializing transformative treatments for patients with serious immunological diseases, today announced data from its ORIGIN Phase 2b trial of atacicept in immunoglobulin A nephropathy (IgAN) that show stabilized kidney function through 96 weeks of long-term follow-up. These data were presented in a late-breaking oral presentation at the American Society of Nephrology Kidney Week 2024 in San Diego, California, and simultaneously published in a manuscript in the Journal of the American Society of Nephrology.
“The 96-week results from the ORIGIN Phase 2b study demonstrated sustained and substantial reductions in Gd-IgA1, hematuria and proteinuria as measured by UPCR with long-term stabilization of eGFR,” said Jonathan Barratt, MD, PhD, FRCP, Mayer Professor of Renal Medicine at the University of Leicester. “Converting patients with IgAN from an eGFR profile of unrelenting decline to a profile consistent with the general population without kidney disease is a differentiated and compelling finding. Collectively, the data support the potential of atacicept to modify the natural history of the disease and prevent kidney failure during the lifetime of patients with IgAN.”
“We are excited to present these long-term efficacy and safety data from the ORIGIN Phase 2b study, which further demonstrate atacicept’s potential to address the underlying pathogenesis of IgAN. The stabilization of kidney function through two years—the longest duration of data among B cell modulators to date—positions atacicept as a potential best- and first-in-class treatment option for patients with IgAN,” said Marshall Fordyce, M.D., Founder and CEO of Vera Therapeutics. “We look forward to announcing expected topline results from the Phase 3 ORIGIN 3 trial in Q2 2025, with planned BLA submission to the U.S. FDA later in the year.”
Over 96 weeks, participants treated with atacicept demonstrated a -
Figure 1. ORIGIN Phase 2b long-term 96-week results with atacicept was consistent with disease-modifying IgAN profile
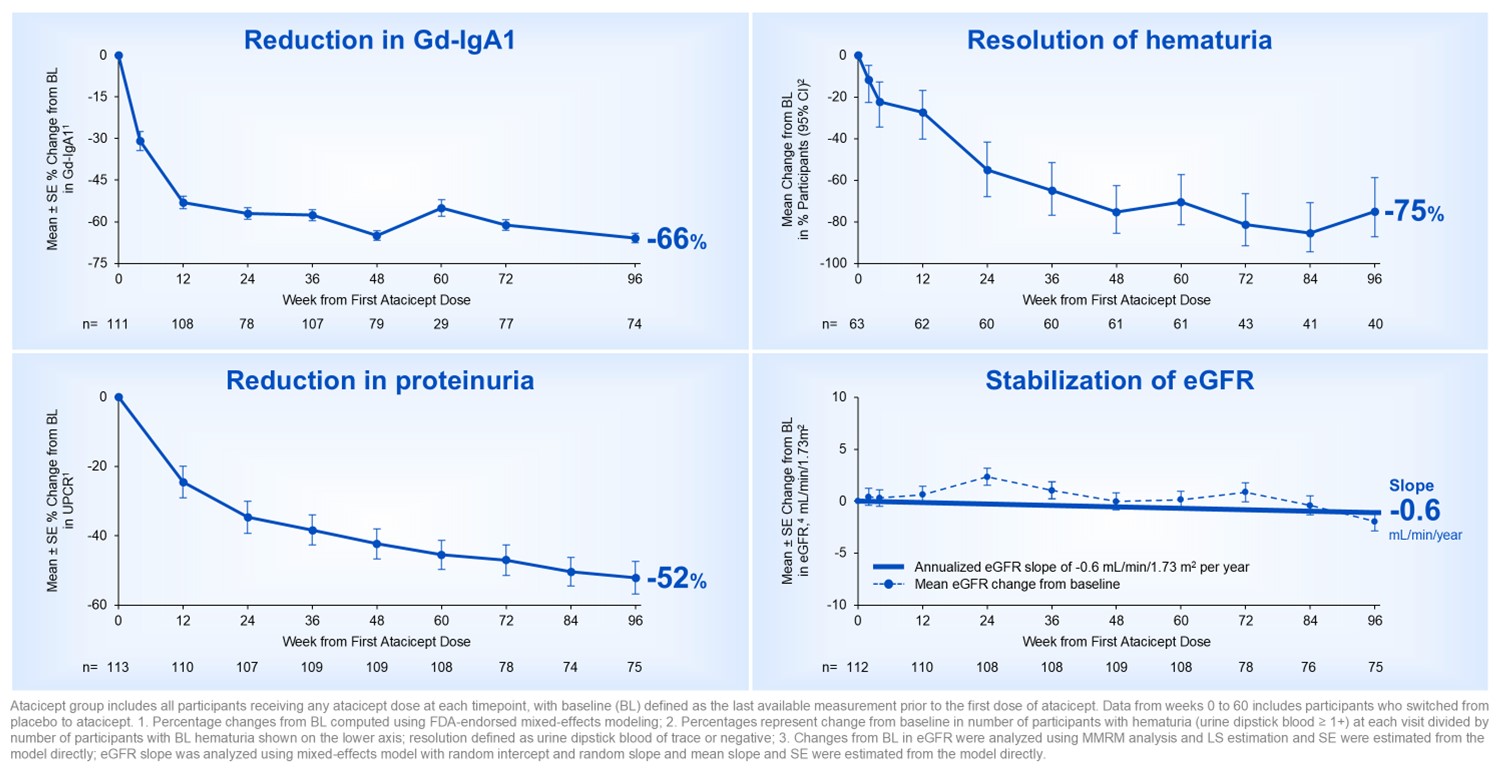
The Company believes these data support the potential for atacicept to offer long-term, comprehensive IgAN disease modification and provide further confidence in the ongoing pivotal Phase 3 ORIGIN 3 trial of atacicept in IgAN.
The Company will host an investor call and webcast to discuss the data update on Monday, October 28, at 8:00 AM ET. The live webcast will be available on the Company’s Investor Calendar at https://ir.veratx.com/news-events/investor-calendar, with the recording and presentation available immediately following the event.
The Kidney Week 2024 presentation and posters are available on the Company’s website at https://ir.veratx.com/news-events/presentations.
Upcoming milestones:
- ORIGIN Extend – plan to initiate a study in Q4 2024 that will provide ORIGIN participants with extended access to atacicept prior to commercial availability in their region, as well as an opportunity to capture longer-term data.
- Pivotal ORIGIN 3 trial on track to announce topline results in Q2 2025, with planned BLA submission to the U.S. FDA later in the year
- PIONEER Study – plan to initiate a study in 2025 that will evaluate the efficacy and safety of atacicept in:
- Expanded IgAN populations – The first set of cohorts will include adults with low kidney function (eGFR 20 to <30 mL/min/1.73 m2), low (UPCR <1.0 g/g) or high proteinuria (UPCR ≥5.0 g/g) or IgAN recurrence after kidney transplant; adolescents at high risk of progression (UPCR ≥0.3 g/g); as well as adolescents and adults with IgA vasculitis nephritis.
- Anti-PLA2R and anti-nephrin podocytopathies – The PIONEER study will expand to additional autoimmune glomerular diseases characterized by the presence of antibodies to glomerular antigens, including primary membranous nephropathy, focal segmental glomerulosclerosis, and minimal change disease.
About the Phase 2b ORIGIN clinical trial
The Phase 2b ORIGIN clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of atacicept in 116 patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of a renin-angiotensin-aldosterone system inhibitor for at least 12 weeks that is the maximum labeled or tolerated dose. The Phase 2b ORIGIN clinical trial evaluated three dose strengths of atacicept versus placebo, administered weekly by prefilled syringe. Patients were randomized 2:2:1:2 to atacicept 150 mg, atacicept 75 mg, atacicept 25 mg or matching placebo. Upon completion of the 36-week blinded treatment period, all patients were offered open-label atacicept 150 mg for an additional 60 weeks.
The primary endpoint was the change in proteinuria as evaluated by urine protein to creatinine ratio (UPCR) at week 24, and the key secondary endpoint was the change in proteinuria as evaluated by UPCR at week 36. Additional exploratory endpoints include change in proteinuria as evaluated by UPCR at weeks 12, 48, and 96; change in eGFR; change in serum immunoglobulin levels, and change in serum Gd-IgA1 levels; safety and tolerability; and serum pharmacokinetics.
The trial met its primary and key secondary endpoints, with statistically significant and clinically meaningful proteinuria reductions and stabilization of eGFR versus placebo through week 36. The safety profile was comparable between atacicept and placebo.
For more information about the Phase 2b ORIGIN clinical trial, please visit www.clinicaltrials.gov.
About the Phase 3 ORIGIN 3 clinical trial
The ORIGIN 3 clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled Phase 3 trial evaluating the safety and efficacy of atacicept in patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of renin-angiotensin system inhibitors for at least 12 weeks that is the maximum labeled or tolerated dose. The objectives of the trial are to determine the effect of atacicept on proteinuria and preservation of kidney function compared to placebo.
For more information about the ORIGIN 3 clinical trial, please visit http://www.clinicaltrials.gov.
About Atacicept
Atacicept is an investigational recombinant fusion protein that contains the soluble transmembrane activator and calcium-modulating cyclophilin ligand interactor receptor that binds to the cytokines B-cell activating factor (BAFF) and A PRoliferation-Inducing Ligand (APRIL). These cytokines are members of the tumor necrosis factor family that promote B-cell survival and autoantibody production associated with certain autoimmune diseases, including IgAN and lupus nephritis.
The Phase 2b ORIGIN clinical trial of atacicept in IgAN met its primary and key secondary endpoints, with statistically significant and clinically meaningful proteinuria reductions and stabilization of eGFR versus placebo through 36 weeks. The safety profile during the randomized period was comparable between atacicept and placebo. Through 72 weeks, atacicept demonstrated further reductions in Gd-IgA1, hematuria and proteinuria, as well as stabilization of eGFR reflecting a profile consistent with that of the general population without IgAN.
Atacicept has received FDA Breakthrough Therapy Designation for the treatment of IgAN, which reflects the FDA’s determination that, based on an assessment of data from the Phase 2b ORIGIN clinical trial, atacicept may demonstrate substantial improvement on a clinically significant endpoint over available therapies for patients with IgAN. Vera believes atacicept is positioned for best-in-class potential, targeting B cells and plasma cells to reduce autoantibodies and having been administered to more than 1,500 patients in clinical studies across different indications.
About Vera
Vera Therapeutics is a late clinical-stage biotechnology company focused on developing treatments for serious immunological diseases. Vera’s mission is to advance treatments that target the source of immunological diseases in order to change the standard of care for patients. Vera’s lead product candidate is atacicept, a fusion protein self-administered as a subcutaneous injection once weekly that blocks both BAFF and APRIL, which stimulate B cells and plasma cells to produce autoantibodies contributing to certain autoimmune diseases, including IgAN, also known as Berger’s disease, and lupus nephritis. In addition, Vera is evaluating additional diseases where the reduction of autoantibodies by atacicept may prove medically useful. Vera is also developing MAU868, a monoclonal antibody designed to neutralize infection with BKV, a polyomavirus that can have devastating consequences in certain settings such as kidney transplant. Vera retains all global developmental and commercial rights to atacicept and MAU868. For more information, please visit www.veratx.com.
Forward-looking Statements
Statements contained in this press release regarding matters, events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include statements regarding, among other things, Vera’s expectations regarding the expansion of its development pipeline for atacicept, atacicept’s potential to be a best-in-class treatment for patients with IgAN, Vera’s expectations regarding the potential for B cell modulation through BAFF/APRIL dual inhibition to transform the treatment landscape for certain autoimmune diseases, Vera’s plans to initiate the ORIGIN Extend study in the fourth quarter of 2024 providing extended access to atacicept to ORIGIN participants, Vera’s plans to initiate the PIONEER study in 2025, Vera’s anticipated presentations of clinical trial data, including the announcement of topline results from the Phase 3 ORIGIN 3 trial in the second quarter of 2025, Vera’s plans for a BLA filing for atacicept in 2025 and Vera’s product candidates, strategy, and regulatory matters. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “expects,” “plan,” “potential,” “will,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon Vera’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks related to the regulatory approval process, results of earlier clinical trials may not be obtained in later clinical trials, preliminary results may not be predictive of topline results, risks and uncertainties associated with Vera’s business in general, the impact of macroeconomic and geopolitical events, and the other risks described in Vera’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management’s assumptions and estimates as of such date. Vera undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by law.
For more information, please contact:
Investor Contact:
Joyce Allaire
LifeSci Advisors
212-915-2569
jallaire@lifesciadvisors.com
Media Contact:
Madelin Hawtin
LifeSci Communications
MHawtin@lifescicomms.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/e8cbc495-7104-43c9-a2ed-6d0e38ad21ae








