OKYO Pharma Announces Promising Categorical Data from OK-101 Phase 2 Trial in Dry Eye Disease
OKYO Pharma has announced promising new data from its Phase 2 trial of OK-101 for treating Dry Eye Disease (DED). The trial, involving 240 patients, showed a 68% improvement in the responder rate for those achieving both conjunctival staining and ocular pain endpoints. OK-101 demonstrated a strong tolerability profile, with favorable comfort scores. The analyses identified conjunctival staining and ocular pain as potential co-primary endpoints for future trials. CEO Gary S. Jacob emphasized these endpoints as important for ongoing development, targeting the underserved DED patient population with pain symptoms.
- 68% improvement in responder rate for conjunctival staining and ocular pain endpoints.
- Favorable tolerability profile and comfort scores for OK-101.
- Identification of potential co-primary endpoints for future trials.
- Only a 19% difference in responder rates for conjunctival staining and blurred vision.
Insights
OKYO Pharma's recent announcement about the categorical data from their Phase 2 trial for OK-101 in dry eye disease (DED) is promising. From a financial perspective, the
However, it is important to recognize that these are still early-stage results. The Phase 2 trial is an important milestone but not a final indicator of commercial success. The next steps will involve larger Phase 3 trials, which are more expensive and time-consuming. Investors should be mindful of the associated costs and the potential for delays, which are common in clinical trials.
Furthermore, conjunctival staining and ocular pain as potential co-primary endpoints might align well with FDA requirements, given that both a 'sign' and a 'symptom' endpoint are needed. However, this path is not free of challenges. Regulatory approval hinges on replicating these positive outcomes in larger trials. A favorable tolerability profile is a positive aspect, reducing risk related to safety concerns, but the competitive landscape should also be considered. There are other companies working on similar treatments and any advancements by competitors could impact OKYO's market potential.
The Phase 2 trial results for OK-101 in treating dry eye disease (DED) are indeed promising from a clinical standpoint. A 68% improvement in the responder rate for patients achieving both the conjunctival staining 'sign' and ocular pain 'symptom' endpoints indicates that OK-101 could address significant unmet medical needs. The
Conjunctival staining measures damage to the eye's conjunctiva, while ocular pain assesses discomfort levels, both of which are critical indicators of DED severity. The data showed a more pronounced improvement in these endpoints with OK-101 compared to the placebo, suggesting a more effective therapeutic action. The smaller improvement in blurred vision indicates some variability in patient responses, but this is not uncommon in DED treatments due to the condition's complexity.
What's equally important is the drug's tolerability profile, with excellent comfort scores for the eye drops. This factor is often overlooked but is important because patient adherence to treatment regimens can be significantly influenced by comfort during application. These results provide a clearer roadmap for future trials, potentially streamlining the pathway to regulatory approval.
In summary, while these findings are promising, they must be validated in larger, more comprehensive Phase 3 trials. The medical community will be looking for consistent efficacy and safety data to fully endorse OK-101 as a new standard in DED therapy.
- Encouraging
68% improvement in responder rate results from patients who achieved both the conjunctival staining “sign” and ocular pain “symptom” endpoints from the 240 patient Phase 2 Dry Eye Disease (DED) trial - Conjunctival staining and ocular pain represent potential co-primary endpoints to be explored in future trials
LONDON and NEW YORK, July 10, 2024 (GLOBE NEWSWIRE) -- OKYO Pharma Limited (NASDAQ: OKYO), a clinical-stage biopharmaceutical company developing innovative ocular therapies for the treatment of inflammatory dry eye disease (DED), a multi-billion-dollar market, and neuropathic corneal pain (NCP), an ocular condition associated with pain but without an FDA approved therapy, today announced promising new categorical data analyses from the recent OK-101 Phase 2 trial in DED patients. These analyses have identified conjunctival staining and ocular pain as the highest potential “sign” and “symptom” co-primary endpoints to be explored in the next DED trial of OK-101.
“The data from this first in-human trial of OK-101 in patients with DED have established a clear road map for future clinical development in this indication,” said Gary S. Jacob, Ph.D., CEO of OKYO Pharma. “Through our analytical work we have concluded that conjunctival staining and ocular pain represent important and de-risked endpoints to be studied further to help underserved patients whose dry eye symptoms include pain component. Furthermore, this trial demonstrated a favorable tolerability profile for OK-101, with an excellent eyedrop comfort score for a topically administered drug.”
“Dry eye disease encompasses a diverse and dissatisfied patient population who needs treatment alternatives to available anti-inflammatory medicines which are insufficient to alleviate the broad spectrum of bothersome ocular symptoms encompassed by this condition,” said Gabriele Cerrone, Non-Executive Chairman of OKYO Pharma. "We continue to advance our innovative program which focuses on the segment of patients most impacted by ocular pain, and will evaluate next steps for dry eye with our advisors and the regulatory agencies.”
Categorical Analysis Details:
Recently released data from the Phase 2, randomized, double-masked, placebo-controlled trial evaluating the safety and efficacy of OK-101 ophthalmic solution in subjects with DED were analyzed by categorical evaluation of the data set and responder-rate analyses. Importantly, the data set for the analysis utilized the full Intent-to-Treat population of 240 patients (for additional details, please refer to slides in the recently issued 6-K filing).
Key findings are highlighted in Figure 1 below which evaluated responder rates for those patients demonstrating at least
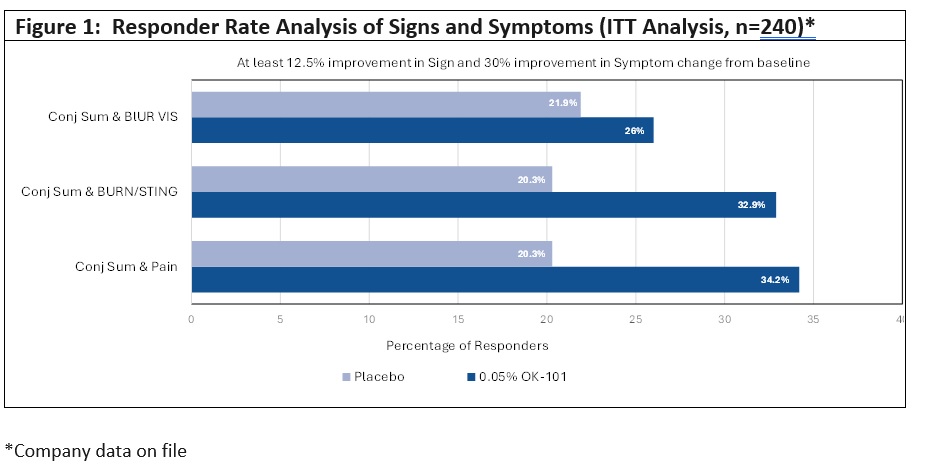
- Notably, the number of patients showing both a reduction in conjunctival sum staining and in the pain symptom in the OK-101-treated group was
34.2% compared to20.3% in the placebo-treated group, a68% improvement. - Similarly, the number of patients with reduction in conjunctival sum staining and burning/stinging symptoms were also numerically higher in the OK-101-treated group (
32.9% ) compared to the placebo-treated group (20.3% ), with a62% improvement. - There was a smaller
19% difference in the responder rates for patients reporting both a combination of conjunctival staining and blurred vision.
FDA requires a “sign” and a “symptom” endpoint in two well controlled registration trials for approval. https://www.fda.gov/media/144594/download
About Dry Eye Disease
Dry eye disease is a common condition that occurs when an individual’s tears are unable to adequately lubricate the eyes. This condition affects approximately 49 million people in the U.S. alone and has been a difficult one to positively diagnose and to treat due to the multifactorial nature of the condition. A number of contributing factors can lead to this condition, including age, sex, certain medical conditions, reduced tear production and tear film dysfunction. Tear film instability typically leads to inflammation and damage to the ocular surface.
About OK-101
OK-101 is a lipid conjugated chemerin peptide agonist of the ChemR23 G-protein coupled receptor which is typically found on immune cells of the eye responsible for the inflammatory response. OK-101 was developed using a membrane-anchored-peptide technology to produce a novel long-acting drug candidate for treating dry eye disease. OK-101 has been shown to produce anti-inflammatory and pain-reducing efficacy signals in mouse models of dry eye disease and corneal neuropathic pain (NCP), respectively, and is designed to combat washout through the inclusion of the lipid anchor built into the drug molecule to enhance the residence time of OK-101 within the ocular environment. OK-101 recently showed statistical significance in multiple endpoints in a recently completed Phase 2, multi-center, double-blind, placebo-controlled trial of OK-101 to treat DED.
About OKYO
OKYO Pharma Limited (NASDAQ: OKYO) is a clinical stage biopharmaceutical company developing innovative therapies for the treatment of DED and NCP, with ordinary shares listed for trading on the NASDAQ Capital Market. OKYO is focused on the discovery and development of novel molecules to treat inflammatory DED and ocular pain. In addition to the recently completed Phase 2 DED trial, OKYO also has plans underway for the opening of a Phase 2 trial for OK-101 to treat NCP in patients with this debilitating condition. For further information, please visit www.okyopharma.com.
Forward-Looking Statements
Certain statements made in this announcement are forward-looking statements. These forward-looking statements are not historical facts but rather are based on the Company’s current expectations, estimates, and projections about its industry, its beliefs, and assumptions. Words such as ‘anticipates,’ ‘expects,’ ‘intends,’ ‘plans,’ ‘believes,’ ‘seeks,’ ‘estimates,’ and similar expressions are intended to identify forward-looking statements. These statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties, and other factors, some of which are beyond the Company’s control, are difficult to predict, and could cause actual results to differ materially from those expressed or forecasted in the forward-looking statements. These and additional risks and uncertainties are described more fully in the company’s filings with the SEC, including those factors identified as “Risk Factors” in our most recent Annual Report on Form 20-F, for the fiscal year ended March 31, 2023. The Company cautions security holders and prospective security holders not to place undue reliance on these forward-looking statements, which reflect the view of the Company only as of the date of this announcement. The forward-looking statements made in this announcement relate only to events as of the date on which the statements are made. The Company will not undertake any obligation to release publicly any revisions or updates to these forward-looking statements to reflect events, circumstances, or unanticipated events occurring after the date of this announcement except as required by law or by any appropriate regulatory authority.
Enquiries:
| OKYO Pharma Limited | Gary S. Jacob, Chief Executive Officer | 917-497-7560 |
| Business Development & Investor Relations | Paul Spencer | +44 (0)20 7495 2379 |
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/eaefc657-3c15-4cbe-9fa5-956b32a662fc








