Orthofix Announces US Launch and First Patient Implants with New fiberFUSE Strip – An Advanced Fiber Bone-Graft Solution Containing Cancellous Bone
Orthofix Medical Inc. (NASDAQ:OFIX) has launched the fiberFUSE™ Strip, a new bone-graft solution for spinal procedures. This innovative product is composed entirely of natural bone and is designed for optimized application in cervical and lumbar surgeries. The fiberFUSE Strip enables cellular ingrowth and new bone formation due to its unique composition. The technology was developed to provide convenience and effectiveness for spine surgeons and their patients. MTF Biologics is the exclusive processor of this product, ensuring high-quality standards through validated aseptic processing.
- Launch of the fiberFUSE Strip may enhance product portfolio and increase sales.
- FiberFUSE Strip is made from 100% natural bone, ensuring a high-quality graft solution.
- Exclusive processing by MTF Biologics may improve product credibility.
- Potential slow adoption by surgeons could hinder initial sales.
- Risk that the product may not improve patient outcomes as anticipated.
Insights
Analyzing...
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced the launch and first patient implants with the fiberFUSE™ Strip, an advanced demineralized fiber bone-graft solution containing cancellous bone. The fiberFUSE Strip is formulated as a convenient preformed bone-graft strip to enable optimized application for posterior cervical, posterior lumbar and degenerative spinal procedures.
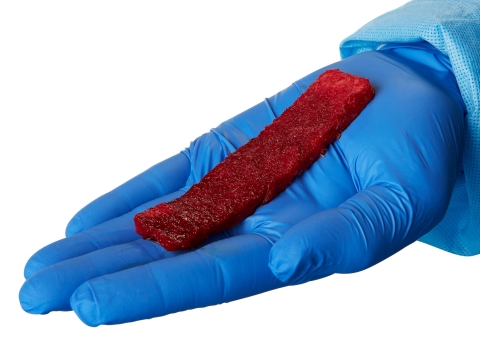
Image of the Orthofix fiberFUSE™ Strip, an advanced demineralized fiber bone-graft solution containing cancellous bone. (Photo: Business Wire)
Comprised of natural 100-percent bone, the fiberFUSE Strip contains a unique mixture of mineralized cancellous and demineralized cortical bone, with no carrier added, to create a natural scaffold allowing for revascularization, cellular ingrowth and new bone formation.
“We are pleased to introduce this next-generation formulation in the fiberFUSE allograft line,” said Kevin Kenny, President of Orthofix Global Spine. “The fiberFUSE Strip delivers a high-quality advanced bone-graft option in a convenient, easy-to-use strip preparation. This technology advancement was developed as part of our strategy to provide procedurally-focused solutions for spine surgeons and their patients.”
MTF Biologics is the exclusive processor of the Orthofix fiberFUSE Strip. Their proprietary, validated aseptic processing methods retain the natural growth factors within the cortical fibers. The fibers interconnect, resulting in a pliable, cohesive graft. The cancellous matrix component provides a porous scaffold to allow ingrowth of host vasculature, osteoblasts and mesenchymal stem cells. The fiberFUSE Strip can be rapidly rehydrated with the surgeon’s reconstitution solution of choice.
About Orthofix
Orthofix Medical Inc. is a global medical device with a spine and orthopedics focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals to improve patient mobility. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedics products are distributed in more than 60 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
About MTF Biologics
MTF Biologics is a global nonprofit organization that saves and heals lives by advancing tissue and organ donation, transplantation, and research. They provide unmatched service, resources, and expertise to donors and their loved ones who give the gift of life, people who depend on tissue and organ transplants, healthcare providers, and clinicians and scientists. MTF Biologics subsidiary, The International Institute for the Advancement of Medicine (IIAM), honors donors of non-transplantable organs by providing their gifts to the medical research community to combat and cure diseases. Their subsidiary, Statline, provides specialized communications and technology expertise to organ, tissue, and eye procurement organizations, as well as the hospitals and patients that they serve. MTF Biologics sister organization, The German Institute for Cell and Tissue Transplantation (Deutsches Institute for Zell-und Gewebeersatz – DIZG) expands their reach to patients across the globe. For more information, visit mtfbiologics.org.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2020 (the “2020 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to: the risk that surgeons may be slow to adopt the fiberFUSE Strip; the risk that future patient studies or clinical experience and data may indicate that treatment with the fiberFUSE Strip does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of its use to patients, hospitals and surgeons; the risk that the product may not perform as intended and may therefore not achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210728005105/en/






