Monopar Receives Clearance to Proceed with Phase 1 Therapeutic Trial of Novel Radiopharmaceutical in Advanced Cancers
Monopar Therapeutics Inc. (Nasdaq: MNPR) has received Human Research Ethics Committee (HREC) clearance in Australia to begin a Phase 1 therapeutic trial of its novel radiopharmaceutical MNPR-101-Lu. This treatment combines lutetium-177 with Monopar's proprietary humanized monoclonal antibody MNPR-101, which targets the urokinase plasminogen activator receptor (uPAR). The trial will enroll patients with advanced solid cancers and follows the ongoing MNPR-101-Zr imaging and dosimetry clinical trial.
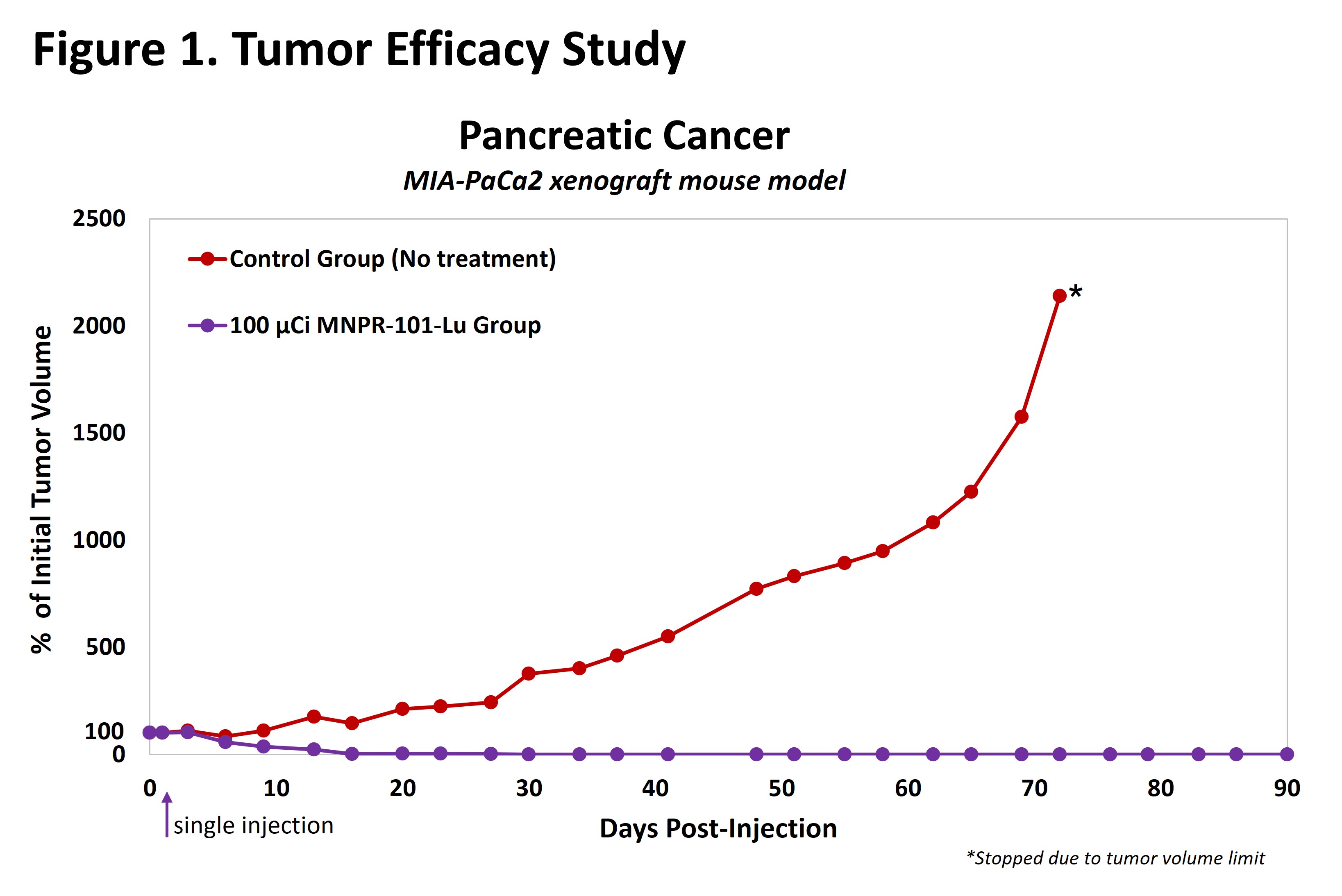
Preclinical studies have shown promising results, with MNPR-101-Lu demonstrating durable antitumor effects in a human pancreatic cancer xenograft mouse model. The treatment achieved complete tumor elimination after a single injection, lasting throughout the 90-day study. Imaging data also revealed high specificity and durable uptake of MNPR-101-Lu in tumors relative to normal tissue.
Monopar Therapeutics Inc. (Nasdaq: MNPR) ha ricevuto l'
Gli studi preclinici hanno mostrato risultati promettenti, con MNPR-101-Lu che dimostra effetti antitumorali duraturi in un modello murino di xeno trapianto di cancro pancreatico umano. Il trattamento ha portato a un'eliminazione completa del tumore dopo una singola iniezione, persistere per tutto il periodo di studio di 90 giorni. I dati di imaging hanno rivelato anche un'alta specificità e un'assorbimento duraturo di MNPR-101-Lu nei tumori rispetto ai tessuti normali.
Monopar Therapeutics Inc. (Nasdaq: MNPR) ha recibido la autorización del Comité de Ética de Investigación Humana (HREC) en Australia para comenzar un ensayo terapéutico de Fase 1 de su nuevo radiofármaco MNPR-101-Lu. Este tratamiento combina lutetio-177 con el anticuerpo monoclonal humanizado de Monopar, MNPR-101, que se dirige al receptor del activador del plasminógeno uroquinasa (uPAR). El ensayo incluirá a pacientes con cáncer sólido avanzado y sigue el ensayo clínico en curso de imagenología y dosimetría MNPR-101-Zr.
Los estudios preclínicos han mostrado resultados prometedores, con MNPR-101-Lu demostrando efectos antitumorales duraderos en un modelo de xeno injerto de cáncer pancreático humano en ratones. El tratamiento logró la eliminación completa del tumor después de una sola inyección, durando a lo largo del estudio de 90 días. Los datos de imagenología también revelaron una alta especificidad y una captación duradera de MNPR-101-Lu en los tumores en comparación con el tejido normal.
Monopar Therapeutics Inc. (Nasdaq: MNPR)는 호주에서 인간 연구 윤리 위원회(HREC)의 승인을 받아 새로운 방사선 의약품 MNPR-101-Lu에 대한 1상 치료 시험을 시작합니다. 이 치료는 루테튬-177과 Monopar의 독점적인 인간화 모노클로날 항체 MNPR-101을 결합하여, 유로키나아제 플라스를노겐 활성화 수용체(uPAR)를 표적으로 합니다. 이 시험은 진행성 고형암 환자를 모집할 예정이며, 현재 진행 중인 MNPR-101-Zr 이미징 및 복용량 연구 임상 시험을 따릅니다.
전임상 연구는 유망한 결과를 보여주었으며, MNPR-101-Lu가 인간 췌장암 이식 모델에서 지속적인 항종양 효과를 나타내었습니다. 이 치료는 단일 주사 후 종양을 완전히 제거하였으며, 90일 동안 지속되었습니다. 이미징 데이터는 또한 종양에 대한 MNPR-101-Lu의 높은 특이성과 지속적인 흡수를 나타냈습니다.
Monopar Therapeutics Inc. (Nasdaq: MNPR) a reçu l'
Les études précliniques ont montré des résultats prometteurs, MNPR-101-Lu démontrant des effets antitumoraux durables dans un modèle de xénogreffe de cancer du pancréas humain. Le traitement a permis une élimination complète de la tumeur après une seule injection, persistant au cours de l'étude de 90 jours. Les données d'imagerie ont également révélé une haute spécificité et une prise durable de MNPR-101-Lu dans les tumeurs par rapport aux tissus normaux.
Monopar Therapeutics Inc. (Nasdaq: MNPR) hat in Australien die Zulassung des Human Research Ethics Committee (HREC) erhalten, um eine Phase-1-Therapiestudie seines neuartigen Radiopharmazeutikums MNPR-101-Lu zu beginnen. Diese Behandlung kombiniert Lutetium-177 mit Monopars proprietärem humanisierten monoklonalen Antikörper MNPR-101, der den Urokinase-Plasminogen-Aktivator-Rezeptor (uPAR) anvisiert. Die Studie wird Patienten mit fortgeschrittenen soliden Tumoren einschließen und folgt der laufenden klinischen Studie zur Bildgebung und Dosimetrie von MNPR-101-Zr.
Präklinische Studien haben vielversprechende Ergebnisse gezeigt, wobei MNPR-101-Lu dauerhafte antitumorale Wirkungen in einem humanen Pankreaskarzinom-Xenotransplantationstiermodell demonstrierte. Die Behandlung erreichte eine vollständige Tumorentfernung nach einer einzigen Injektion, die über die 90-tägige Studie hinweg anhaltend war. Bildgebungsdaten zeigten auch eine hohe Spezifität und eine langfristige Akkumulation von MNPR-101-Lu in Tumoren im Vergleich zu normalem Gewebe.
- HREC clearance received to commence Phase 1 therapeutic trial of MNPR-101-Lu
- Preclinical studies showed complete tumor elimination in pancreatic cancer mouse model
- High specificity and durable uptake of MNPR-101-Lu in tumors demonstrated in imaging data
- Potential to treat aggressive cancers like triple negative breast cancer and pancreatic cancer
- None.
Insights
Monopar's HREC clearance for the Phase 1 trial of MNPR-101-Lu is a significant milestone in radiopharmaceutical development. The combination of Lu-177 with the uPAR-targeting antibody MNPR-101 shows promising preclinical results, particularly in pancreatic cancer models. The reported complete tumor elimination and durable antitumor effects in xenograft studies are noteworthy, suggesting potential efficacy against aggressive cancers.
However, investors should note that preclinical success doesn't guarantee clinical efficacy. The upcoming Phase 1 trial will be important in assessing safety and initial efficacy in humans. The focus on uPAR-positive tumors, including triple-negative breast and pancreatic cancers, targets areas of high unmet medical need, potentially enhancing the therapy's market value if successful.
This HREC clearance represents a positive development for Monopar, potentially accelerating their clinical pipeline. The progression to a therapeutic trial following the ongoing imaging study demonstrates efficient resource utilization and a step-wise approach to drug development. However, as a clinical-stage company, Monopar's financial runway and burn rate remain critical factors for investors to monitor.
The targeting of aggressive cancers with high unmet needs could position MNPR-101-Lu for expedited regulatory pathways if early results are promising, potentially shortening time to market. Investors should watch for updates on trial initiation, enrollment progress and any preliminary data readouts, as these will be key catalysts for the stock in the near to medium term.
WILMETTE, Ill., Aug. 21, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR), a clinical-stage radiopharma company focused on developing innovative treatments for cancer patients, today announced it has received Human Research Ethics Committee (HREC) clearance in Australia to commence a Phase 1 therapeutic trial of its novel radiopharmaceutical MNPR-101-Lu.
MNPR-101-Lu combines the therapeutic radioisotope lutetium-177 (Lu-177) with Monopar’s proprietary first-in-class humanized monoclonal antibody MNPR-101, which is highly selective against the urokinase plasminogen activator receptor (uPAR). The MNPR-101-Lu Phase 1 clinical trial will enroll patients with advanced solid cancers and will be a therapeutic follow-on study to the currently ongoing MNPR-101-Zr imaging and dosimetry clinical trial.
The results from preclinical studies of MNPR-101-Lu are promising. In a 90-day efficacy study in a human pancreatic cancer xenograft mouse model (Figure 1, below) as an example, MNPR-101-Lu demonstrated durable antitumor effects after a single injection, achieving complete elimination of tumors that lasted the duration of the study.
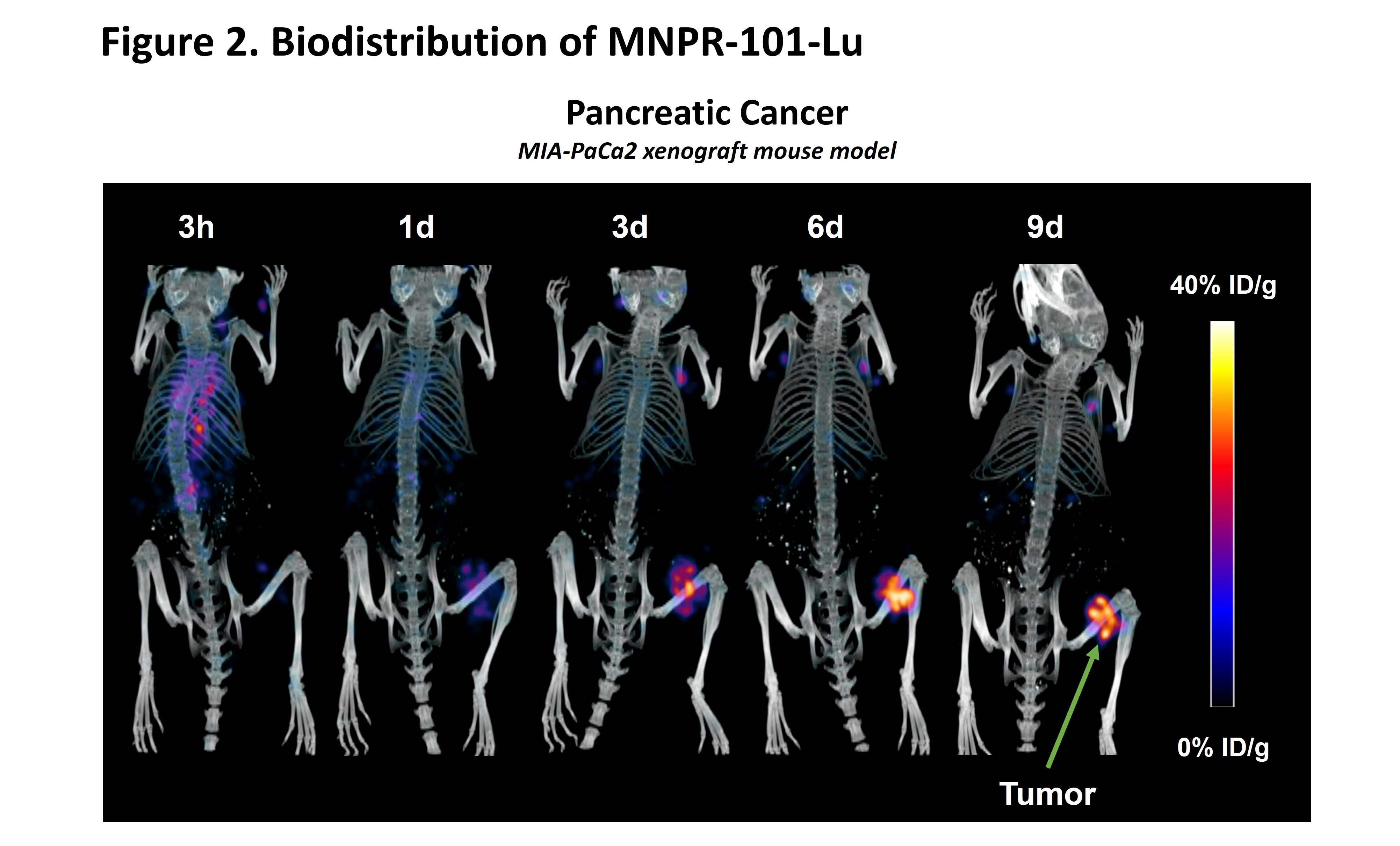
The MNPR-101-Lu imaging data in a human pancreatic cancer xenograft mouse model presented in March (link, Figure 2 below) provides additional insight into the strong therapeutic effect observed after a single injection of MNPR-101-Lu. The imaging data demonstrates the high specificity and durable uptake of MNPR-101-Lu in the tumor relative to normal tissue.
“We are excited about the HREC clearance and encouraged by the potential of MNPR-101-Lu to provide a meaningful clinical benefit to patients with uPAR-positive tumors. Several of the most aggressive, deadly cancers express uPAR, including triple negative breast cancer and pancreatic cancer,” said Chandler Robinson, MD, Monopar’s Chief Executive Officer. “We are looking forward to launching the trial as quickly as we can.”
About Monopar Therapeutics Inc.
Monopar Therapeutics is a clinical-stage radiopharmaceutical company focused on developing innovative treatments for cancer patients, including Phase 1-stage MNPR-101-Zr for imaging advanced cancers and late preclinical-stage MNPR-101-Lu and MNPR-101-Ac225 for the treatment of advanced cancers, as well as early development programs against solid cancers. For more information, visit: www.monopartx.com.
Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of these forward-looking statements include: that the MNPR-101-Lu Phase 1 clinical trial will enroll patients with advanced solid cancers, and will be a therapeutic follow-on to the currently ongoing MNPR-101-Zr imaging and dosimetry clinical trial; the results from preclinical studies of MNPR-101-Lu are promising; that these data support the potential of MNPR-101-Lu to provide a meaningful clinical benefit to patients with uPAR-positive tumors; and that Monopar is looking forward to launching the trial as quickly as it can. The forward-looking statements involve risks and uncertainties including, but not limited to: that Monopar may not launch its MNPR-101-Lu therapeutic study even after receiving regulatory clearance; that the Phase 1 imaging and dosimetry clinical trial in advanced cancer patients with MNPR-101-Zr may not yield satisfactory results, if at all; that future preclinical or clinical data will not be as promising as the data to date; that MNPR-101-Zr and/or MNPR-101-Lu may cause unexpected serious adverse effects or fail to image or be effective against the cancer tumors in humans; and the significant general risks and uncertainties surrounding the research, development, regulatory approval, and commercialization of imaging agents and therapeutics. Actual results may differ materially from those expressed or implied by such forward-looking statements. Risks are described more fully in Monopar's filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made. Monopar undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Any forward-looking statements contained in this press release represent Monopar’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
CONTACT:
Monopar Therapeutics Inc.
Investor Relations
Karthik Radhakrishnan
Chief Financial Officer
karthik@monopartx.com
Follow Monopar on social media for updates:
Twitter: @MonoparTx LinkedIn: Monopar Therapeutics
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/46065fd5-9a23-4688-9bbe-1bdb6e74954e
https://www.globenewswire.com/NewsRoom/AttachmentNg/6fe00a55-f6db-4fba-a0b3-6d4f474b517c

FAQ
What is MNPR-101-Lu and how does it work?
What stage of development is MNPR-101-Lu currently in?
What were the results of MNPR-101-Lu in preclinical studies?







