3M Health Care introduces new 3M SoluPrep S Sterile Antiseptic Solution, an optimized skin prep product for presurgical applications
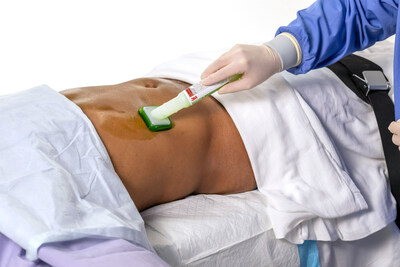
3M Health Care announced the FDA approval of its 3M™ SoluPrep™ S Sterile Antiseptic Solution, which combines chlorhexidine gluconate (2% w/v) and isopropyl alcohol (70% v/v) for patient preoperative skin preparation. This solution demonstrates fast-acting, broad-spectrum antimicrobial activity and lasts for at least 96 hours post-application. The commercial release began on April 3, 2023, with full launch expected in 2024. Key features include a visible Brite Green tint for better coverage, an efficient applicator, and improved adherence of incise drapes. This product aims to enhance safety and confidence for healthcare providers.
- FDA approval of 3M SoluPrep S enhances product portfolio.
- Offers broad-spectrum antimicrobial activity with 96-hour efficacy.
- Features user-friendly applicator for improved workflow efficiency.
- None.
Insights
Analyzing...
"3M SoluPrep S Sterile Antiseptic Solution delivers the efficacy health care providers can depend on, with the same active ingredients as the leading competitor plus added benefits to help protect patients," said Dr.
3M SoluPrep S Sterile Antiseptic Solution's optimized features and functionalities include:
- Brite Green tint, which can be seen on a variety of skin tones, to help users ensure the surgical prep area is covered completely.1
- Designed with work flow efficiency and convenience in mind, the applicator provides greater coverage area compared to the leading CHG/IPA surgical prep product with the same size applicator.1
- Applicator activation is easy and quick, and the foam sponge controls the flow of solution during application to minimize drip. 1
- Contains an ingredient that can improve incise drape adherence.2
A limited commercial release of 3M SoluPrep S Sterile Antiseptic Solution will begin in the
For more information on 3M SoluPrep S Sterile Antiseptic Solution, visit www.3M.com/SoluPrepS.
The 3M Medical Solutions Division skin and wound care portfolio includes a comprehensive range of integrated surgical and skin care solutions -- advanced and acute wound care dressings and products, medical tapes, sterilization products, patient prep and warming products, advanced wound care and specialty surgical applications, etc. – across the care continuum, from products that protect and maintain the integrity of skin to a full portfolio of advanced surgical wound care solutions.
1 3M Data on File.
2 Olson L, Morse D, Paulson J, Bernatchez S. Evaluation of Incise Drape Lift Using
About 3M
At 3M (NYSE: MMM), we apply science in collaborative ways to improve lives daily as our employees connect with customers all around the world. Learn more about 3M's creative solutions to global challenges at www.3M.com or on Twitter @3M or @3MNews.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/3m-health-care-introduces-new-3m-soluprep-s-sterile-antiseptic-solution-an-optimized-skin-prep-product-for-presurgical-applications-301787866.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/3m-health-care-introduces-new-3m-soluprep-s-sterile-antiseptic-solution-an-optimized-skin-prep-product-for-presurgical-applications-301787866.html
SOURCE 3M