Milestone Pharmaceuticals Announces Presentation of Heart Rate Analysis of NODE-301 Trial of Etripamil in Patients with PSVT
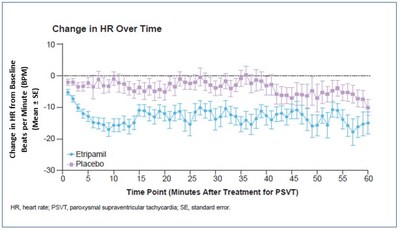
Milestone Pharmaceuticals (Nasdaq: MIST) announced significant findings from a post-hoc analysis of the Phase 3 NODE-301 trial for etripamil, a novel nasal spray treatment for paroxysmal supraventricular tachycardia (PSVT). The analysis revealed that etripamil significantly reduced heart rates during SVT episodes within 5 minutes, sustained for up to 60 minutes. Notably, patients experienced greater heart rate reductions compared to placebo (p<0.0001). These results, presented at the AHA Scientific Sessions 2021, support etripamil’s potential as an effective at-home treatment for PSVT and possibly atrial fibrillation with rapid ventricular response.
- Significant reduction in heart rate observed in patients using etripamil compared to placebo (p<0.0001).
- Rapid heart rate reduction within 5 minutes of administration, sustained for up to 60 minutes.
- Potential for etripamil to be an effective treatment for PSVT in medically unsupervised settings.
- NODE-301 trial did not achieve its primary endpoint regarding time to conversion of SVT to sinus rhythm (p=0.12).
- Limited statistical power due to a small number of placebo patients and prolonged measurement periods.
Insights
Analyzing...
- Etripamil significantly decreased heart rate during SVT episodes and independent of conversion to SR –
- HR changes occurred within 5 minutes and were sustained for 60 minutes –
- Data presented at AHA Scientific Sessions 2021 -
MONTREAL and CHARLOTTE, N.C., Nov. 15, 2021 /PRNewswire/ -- Milestone Pharmaceuticals Inc. (Nasdaq: MIST), a biopharmaceutical company focused on the development and commercialization of innovative cardiovascular medicines, today announced new data from a post-hoc analysis of the Phase 3, randomized, double-blind, placebo-controlled NODE-301 trial of etripamil nasal spray, the Company's novel investigational, short-acting calcium channel blocker, in patients with paroxysmal supraventricular tachycardia (PSVT). The analysis demonstrated that etripamil significantly decreased heart rate (HR) prior to conversion to sinus rhythm (SR). The data were featured in a poster presentation titled, "Etripamil Nasal Spray Reduces Heart Rate in Patients with Paroxysmal Supraventricular Tachycardia Prior to Conversion to Sinus Rhythm," at the American Heart Association (AHA) Scientific Sessions 2021 held November 13-15, 2021.
"Data reported in this analysis align with the potential of etripamil to serve as an effective treatment option for patients with PSVT in the medically-unsupervised setting," said James Ip, M.D., Associate Professor of Clinical Medicine, Division of Cardiology, Weill Cornell Medicine, New York Presbyterian Hospital. "Beyond PSVT, these findings provide a strong impetus for the continued assessment of etripamil in other conditions requiring ventricular rate reduction, such as atrial fibrillation with rapid ventricular response (AFib-RVR)."
"I would like to congratulate the study authors and thank our team, the study investigators, and the clinical site colleagues for their hard work in making this analysis possible, and would also like to extend a special thank you to the patients for supporting the etripamil development initiative," said Joseph Oliveto, President and Chief Executive Officer of Milestone Pharmaceuticals. "We remain on track to report topline data from the RAPID trial in the second half of 2022, and in parallel, continue to advance the entire etripamil development program. We are committed to elucidating the full potential of etripamil to treat symptomatic episodes of elevated HR associated with these conditions in the at-home setting."
The NODE-301 trial, which enrolled a total of 431 patients across 65 sites in the U.S. and Canada, was an event-driven, Phase 3 efficacy trial of etripamil versus placebo for terminating supraventricular tachycardia (SVT) episodes in a medically-unsupervised setting. The analysis assessed the effect of etripamil on HR during the SVT episode and prior to conversion to SR, as well as the correlation of HR with patient-reported outcomes in 150 patients (etripamil, n=102; placebo, n= 48). Patients treated with etripamil experienced a significantly greater reduction in mean HR from baseline within 10 minutes of administration as compared to placebo-treated patients (p<0.0001). Self-administration of intranasal etripamil rapidly and significantly decreased HR prior to conversion of SVT to SR, starting within 3 minutes (p<0.03) and lasting up to 40 minutes after treatment (p<0.004), with the trend sustained over the 60-minute observation period. Patients presented non-statistically different baseline HRs in the etripamil (179 beats per minute) and placebo (174 beats per minute) groups. The average drop in HR was modest in the placebo group and was significantly greater (p<0.0001) in the etripamil group over one hour with a maximum of 16 beats per minute between the groups at 10 minutes. Maximal change in HR from baseline was positively correlated with patient-reported relief of symptoms (p=0.0027) and treatment effectiveness (p=0.0034). Further, the observed reduction in HR was independent of conversion to SVT, suggesting the potential utility of etripamil to alleviate PSVT symptoms prior to conversion to SR.
A copy of the presentation is available in the Publications section of Milestone Pharmaceuticals' website.
About NODE-301
NODE-301 is a Phase 3, multicenter, randomized (2:1), double-blind, placebo-controlled, single-administration study of etripamil nasal spray in patients with PSVT. The study targeted a total of 150 adjudicated SVT events. Top line results were reported in March 2020. Despite early activity at 30 minutes, a time period consistent with the relevant pharmacodynamic effect of etripamil, the study did not achieve its primary endpoint of time to conversion of SVT to sinus rhythm (SR) compared to placebo over the pre-specified five-hour period following study drug administration (p=0.12). The small number of placebo patients and prolonged efficacy measurement period was found to have confounded the statistical analysis of the results. The study did demonstrate statistically significant improvements in favor of etripamil over placebo in the important secondary endpoint of patient-reported treatment satisfaction, as well as a trend toward a reduction in emergency department visits. The Company believes the safety and tolerability data from the NODE-301 study is supportive of use of etripamil in a medically-unsupervised setting, with adverse events consistent with those observed in prior trials.
The primary endpoint of the NODE-301 study is time to conversion of an SVT episode to sinus rhythm after the administration of study drug, as confirmed by a central, independent adjudication committee. Secondary study endpoints include relief of symptoms commonly associated with an episode of SVT such as heart palpitations, chest pain, anxiety, shortness of breath, dizziness, or fainting, and rating of treatment satisfaction questionnaire for medication (TSQM).
About Paroxysmal Supraventricular Tachycardia
Paroxysmal supraventricular tachycardia (PSVT) is a condition characterized by intermittent episodes of rapid heartbeat that starts and stops suddenly and without warning that affects approximately two million Americans. Episodes of SVT are often associated with symptoms including palpitations, sweating, chest pressure or pain, shortness of breath, sudden onset of fatigue, lightheadedness or dizziness, fainting, and anxiety. Certain medications, including adenosine, beta-blockers or calcium channel blockers, have long been used for the acute treatment of PSVT. However, these medications must be administered intravenously and under medical supervision, usually in an emergency department or other acute care setting.
About Atrial Fibrillation with Rapid Ventricular Rate
Atrial fibrillation (AFib) is a common arrhythmia marked by an irregular and often rapid heartbeat. AFib is estimated to affect five million patients in the United States, a prevalence projected by the Centers for Disease Control to increase to twelve million patients within the next 10 years. Atrial fibrillation with rapid ventricular rate (AFib-RVR) is a condition in which patients with AFib experience episodes of abnormally high heart rate, often with symptoms such as palpitations, shortness of breath, dizziness, and weakness. Oral calcium channel blockers and/or beta blockers are commonly used to manage the heart rate in this condition. When episodes do occur, the corresponding symptoms often cause patients to seek care in the acute care setting such as the emergency department, where standard of care procedures include intravenous administration of calcium channel blockers or beta blockers under medical supervision. Milestone's initial qualitative market research indicates that approximately
About Etripamil
Etripamil, Milestone's lead investigational product, is a novel calcium channel blocker designed to be a rapid-response therapy for episodic cardiovascular conditions. As a nasal spray that is self-administered by the patient, etripamil has the potential to shift the current treatment experience for many patients from the emergency department to a medically-unsupervised setting. Milestone is conducting a comprehensive development program for etripamil, with Phase 3 trials ongoing in paroxysmal supraventricular tachycardia (PSVT) and now with a Phase 2 proof-of-concept trial underway in patients with atrial fibrillation with rapid ventricular rate (AFib-RVR).
About Milestone Pharmaceuticals
Milestone Pharmaceuticals Inc. (Nasdaq: MIST), is a biopharmaceutical company focused on the development and commercialization of innovative cardiovascular medicines. Milestone's lead product candidate etripamil is currently in a Phase 3 clinical-stage program for the treatment of paroxysmal supraventricular tachycardia (PSVT) and in a Phase 2 proof-of-concept trial for the treatment of patients with atrial fibrillation and rapid ventricular rate (AFib-RVR). Milestone Pharmaceuticals operates in Canada and the United States. For more information, visit www.milestonepharma.com and follow the Company on Twitter at @MilestonePharma.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as "believe," "may," "will," "expect," "continue," "estimate," "potential," "progress" and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements are based on Milestone's expectations and assumptions as of the date of this press release. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from these forward-looking statements. Forward-looking statements contained in this press release include statements regarding the potential of etripamil as a promising therapy for PSVT patients, the design, progress, timing, scope and results of the RAPID and ReVeRA trials, Milestone's ability to execute on the remainder of the PSVT program, Milestone's ongoing plans to study etripamil in atrial fibrillation patients and estimates about the addressable market and commercial potential for treatments of atrial fibrillation with rapid ventricular rate. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, but are not limited to, the risks inherent in biopharmaceutical product development and clinical trials, including the lengthy and uncertain regulatory approval process, uncertainties related to the timing of initiation, enrollment, completion and evaluation of clinical trials, and whether the clinical trials will validate the safety and efficacy of etripamil for PSVT or other indications, among others, as well as risks related to pandemics and public health emergencies, including those related to the ongoing COVID-19 pandemic, and risks related the sufficiency of Milestone's capital resources and its ability to raise additional capital. These and other risks are set forth in Milestone's filings with the U.S. Securities and Exchange Commission, including in its annual report on Form 10-K for the year ended December 31, 2020, under the caption "Risk Factors." Except as required by law, Milestone assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
Contact:
David Pitts
Argot Partners
212-600-1902
david@argotpartners.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/milestone-pharmaceuticals-announces-presentation-of-heart-rate-analysis-of-node-301-trial-of-etripamil-in-patients-with-psvt-301423722.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/milestone-pharmaceuticals-announces-presentation-of-heart-rate-analysis-of-node-301-trial-of-etripamil-in-patients-with-psvt-301423722.html
SOURCE Milestone Pharmaceuticals, Inc.