Moleculin Announces Presentation of Positive Data Demonstrating High Anti-Cancer Activity of Annamycin and Non-Cardiotoxic Properties
- None.
- None.
Insights
Annamycin's more potent inhibition of topoisomerase II, particularly the beta isoform, is a significant finding in oncology pharmacology. Topoisomerase II enzymes are critical for DNA replication and cell division and their inhibition is a common strategy in cancer treatment. However, the therapeutic window is often narrow due to off-target effects, including cardiotoxicity. The lack of cardiotoxic events in the Annamycin clinical trials could indicate a breakthrough in the development of safer anthracyclines.
From the perspective of drug development, the ability to offer a treatment without the associated risk of cardiotoxicity could lead to higher adoption rates among clinicians and potentially, a larger market share. This is particularly relevant in cancer types where anthracyclines are the standard of care, but their use is limited by their side effects. The patent issuance further strengthens Moleculin's intellectual property position, potentially increasing its valuation and attractiveness to investors.
The clinical implications of Annamycin's lack of cardiotoxicity are profound. Cardiotoxicity is a major concern with anthracyclines and it can lead to long-term health issues for cancer survivors. Annamycin's safety profile could make it a preferred option for treating cancers like AML and STS, particularly in pediatric patients who are especially vulnerable to the long-term effects of cardiotoxic drugs. The focus on topoisomerase II-beta as a mechanism to reduce cardiotoxicity is an innovative approach that may shift treatment paradigms.
For patients, this could mean more effective treatment with fewer long-term cardiovascular risks. For healthcare providers, it could result in fewer complications and associated costs. These factors could influence insurance coverage decisions and ultimately impact Moleculin's market potential.
The announcement of positive preclinical data and the issuance of a composition of matter patent for Annamycin could be a catalyst for Moleculin Biotech's stock performance. Investors often look for such milestones as indicators of a company's future revenue potential and market exclusivity. The lack of cardiotoxicity in a class of drugs known for this side effect could position Annamycin as a disruptor in the oncology market.
Moreover, the patent protection may provide Moleculin with a competitive edge and the ability to negotiate licensing deals or partnerships. The potential expansion into broader patient populations represents a significant growth opportunity. However, investors should consider the regulatory pathway ahead and the costs associated with further development when evaluating the company's long-term potential.
Annamycin demonstrated to be a more potent inhibitor of topoisomerase II-alpha and II-beta while remaining inactive against established cardiomyocyte cultures
Results clearly aligned with lack of drug-related cardiotoxic events in patients treated with Annamycin in ongoing clinical trials;
A prominent treatment for many adult cancers and approximately
Annamycin composition of matter patent issued April 9th; Patent enables expansion into greater patient populations where cardiotoxicity remains an unmet need
The poster titled, Non-cardiotoxic Properties of Annamycin, a Clinically Evaluated Anthracycline and Potent Topoisomerase 2β Poison, was presented in the "Late-Breaking Research: Experimental and Molecular Therapeutics 2" session held on Monday, April 8th. The presented poster outlined results from the assessment and comparison of the potency of doxorubicin (a commonly prescribed anthracycline) and Annamycin, Moleculin's next-generation anthracycline, against topoisomerase II-alpha and II-beta and determine their impact on physiology of human cardiomyocytes demonstrating no pathologic changes in mice hearts following chronic in vivo exposure.
"Cardiotoxicity continues to be a significant side effect limiting the clinical use of anthracyclines, despite anthracyclines representing some of the most important treatments available for AML and Advanced STS. These data, documenting lack of cardiotoxicity of Annamycin and aligned with the data demonstrated to date in our ongoing clinical trials, bolster our confidence in Annamycin potential to offer patients a safe and effective treatment option. Interestingly, the presented data demonstrates that Annamycin appears to be a significantly more potent inhibitor of topoisomerase II-beta than doxorubicin providing validation for additional studies to reevaluate the role of topoisomerase II-beta in anthracycline induced cardiotoxicity," commented Walter Klemp, Chairman and Chief Executive Officer of Moleculin.
"Additionally, with our recently issued patent, which covers composition of matter protection across all indications, we have the potential to expand the development of Annamycin into greater patient populations in indications where cardiotoxicity remains a significant unmet need. We remain highly encouraged by Annamycin and committed to advancing its development," concluded Mr. Klemp.
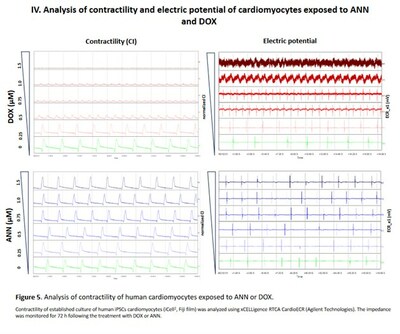
As part of the presented data, the potency of Annamycin and doxorubicin to inhibit topoisomerase II was tested in DNA relaxation assays using recombinant topoisomerase II-alpha and II-beta with kinetoplast as the DNA substrate. Annamycin and doxorubicin cytotoxicity was assessed in a panel of cancer cell lines cardiomyocytes (murine and human). In addition, the effects on cardiomyocyte physiology (beating rate, contraction, electric potential,) were assessed in human cardiomyocytes using the xCELLigence RTCA CardioECR. The pathophysiology of the heart after chronic exposure to the drugs was also evaluated in mice models.
Key Data Highlights:
- Annamycin demonstrated to be a more potent topoisomerase II-alpha and II-beta poison than doxorubicin.
- In contrast to doxorubicin:
- Annamycin does not affect viability of established culture of human iPSCc cardiomyocytes (tested up to 1.5 μM)
- Annamycin does not affect contractility or the electric potential of the cardiomyocytes
- Rat cardiomyocytes (H9c2) appear to be resistant to ANN while sensitive to DOX
- Annamycin is well tolerated by the animals even at schedules exceeding the therapeutic dosage while ex vivo pathology examination of the mice confirmed no toxicity to the heart/myocardium.
- These data are clearly aligned with the lack of drug-related cardiotoxic events in Annamycin-treated patients in ongoing clinical trials
- The role of topoisomerase II-beta in cardiotoxicity of anthracyclines should be further investigated
- A video on the comparison of cardiotoxic effects of Annamycin and doxorubicin on human cardiomyocytes can be accessed here
The Company also announced that the United States Patent and Trademark Office (USPTO) formally issued
Annamycin is currently being evaluated in ongoing clinical trials for the treatment of relapsed or refractory acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases. For more information about the ongoing trials, please visit clinicaltrialsregister.eu: EudraCT 2020-005493-10 or clinicaltrials.gov: NCT05319587; and clinicaltrials.gov: NCT04887298, respectively.
About Moleculin Biotech, Inc.
Moleculin Biotech, Inc. is a clinical stage pharmaceutical company with a growing pipeline, including Phase 2 clinical programs, for hard-to-treat tumors and viruses. The Company's lead program, Annamycin is a next-generation anthracycline designed to avoid multidrug resistance mechanisms with little to no cardiotoxicity. Annamycin is currently in development for the treatment of relapsed or refractory acute myeloid leukemia (AML) and soft tissue sarcoma (STS) lung metastases.
Additionally, the Company is developing WP1066, an Immune/Transcription Modulator capable of inhibiting p-STAT3 and other oncogenic transcription factors while also stimulating a natural immune response, targeting brain tumors, pancreatic and other cancers, and WP1220, an analog to WP1066, for the topical treatment of cutaneous T-cell lymphoma. Moleculin is also engaged in the development of a portfolio of antimetabolites, including WP1122 for the potential treatment of viruses, as well as cancer indications including brain tumors, pancreatic and other cancers.
For more information about the Company, please visit www.moleculin.com and connect on Twitter, LinkedIn and Facebook.
Forward-Looking Statements
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, the expected issuance of the patent discussed above, the pace of enrollment in Moleculin's clinical trials, the timing of Moleculin's ability to report topline data from its studies, the timing of the commencement of investigator-sponsored and/or externally funded clinical trials which are outside the control of Moleculin, and whether the results of Moleculin's preclinical animal models can be replicated in human trials. Although Moleculin believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Moleculin has attempted to identify forward-looking statements by terminology including 'believes,' 'estimates,' 'anticipates,' 'expects,' 'plans,' 'projects,' 'intends,' 'potential,' 'may,' 'could,' 'might,' 'will,' 'should,' 'approximately' or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under Item 1A. "Risk Factors" in our most recently filed Form 10-K filed with the Securities and Exchange Commission (SEC) and updated from time to time in our Form 10-Q filings and in our other public filings with the SEC. Any forward-looking statements contained in this release speak only as of its date. We undertake no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
Investor Contact:
JTC Team, LLC
Jenene Thomas
(833) 475-8247
MBRX@jtcir.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-presentation-of-positive-data-demonstrating-high-anti-cancer-activity-of-annamycin-and-non-cardiotoxic-properties-302112418.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/moleculin-announces-presentation-of-positive-data-demonstrating-high-anti-cancer-activity-of-annamycin-and-non-cardiotoxic-properties-302112418.html
SOURCE Moleculin Biotech, Inc.
FAQ
What positive preclinical data was announced by Moleculin Biotech, Inc. regarding Annamycin at the AACR Annual Meeting?
What is the significance of Annamycin's potency against topoisomerase II-alpha and II-beta?
What does the recent patent issuance enable for Moleculin Biotech, Inc. regarding Annamycin?
In which ongoing clinical trials is Annamycin being evaluated?