Lexaria's Technology Supports Higher Levels of the GLP-1 Drug Semaglutide in Brain
Lexaria Bioscience (Nasdaq:LEXX) has announced positive results from its fluorescently tagged semaglutide (FTS) rodent biodistribution study, demonstrating that its DehydraTECH technology enhances brain delivery of the GLP-1 drug semaglutide.
The study revealed that DehydraTECH-FTS showed higher brain biodistribution compared to Rybelsus® equivalent composition, with the 5mg DehydraTECH-FTS achieving higher brain semaglutide fluorescent signal intensity than the 15mg Rybelsus® equivalent. The enhanced brain delivery could potentially improve both safety and efficacy of GLP-1 drugs, particularly in areas related to appetite suppression and nausea reduction.
These findings suggest that Lexaria's technology may enable unique delivery enhancements in brain tissue, potentially supporting improved pharmacodynamic performance of GLP-1 drugs.
Lexaria Bioscience (Nasdaq:LEXX) ha annunciato risultati positivi dal proprio studio di biodistribuzione sui roditori con semaglutide etichettato fluorescentemente (FTS), dimostrando che la tecnologia DehydraTECH aumenta l'apporto al cervello del farmaco GLP-1 semaglutide.
Lo studio ha rilevato che la DehydraTECH-FTS mostra una maggiore biodistribuzione cerebrale rispetto alla composizione equivalente di Rybelsus®, con la DehydraTECH-FTS da 5 mg che raggiunge una maggiore intensità del segnale fluorescente cerebrale della semaglutide rispetto all'equivalente di 15 mg di Rybelsus®. L'incrementata consegna al cervello potrebbe potenzialmente migliorare sia la sicurezza che l'efficacia dei farmaci GLP-1, in particolare nelle aree legate alla soppressione dell'appetito e alla riduzione della nausea.
Questi risultati suggeriscono che la tecnologia di Lexaria potrebbe consentire miglioramenti di consegna unici nel tessuto cerebrale, potenzialmente supportando una migliore performance farmacodinamica dei farmaci GLP-1.
Lexaria Bioscience (Nasdaq:LEXX) ha anunciado resultados positivos de su estudio de biodistribución en roedores con semaglutida etiquetada fluorescentemente (FTS), demostrando que su tecnología DehydraTECH mejora la entrega al cerebro del fármaco GLP-1 semaglutida.
El estudio mostró que la DehydraTECH-FTS presentó una mayor biodistribución cerebral en comparación con la composición equivalente de Rybelsus®, con la DehydraTECH-FTS de 5 mg logrando una mayor intensidad de la señal fluorescente en el cerebro de semaglutida que la equivalente de 15 mg de Rybelsus®. Esta entrega mejorada al cerebro podría, potencialmente, mejorar tanto la seguridad como la eficacia de los fármacos GLP-1, especialmente en áreas relacionadas con la supresión del apetito y la reducción de las náuseas.
Estos hallazgos sugieren que la tecnología de Lexaria podría permitir mejoras únicas en la entrega al tejido cerebral, apoyando potencialmente un mejor rendimiento farmacodinámico de los fármacos GLP-1.
Lexaria Bioscience (나스닥:LEXX)가 형광으로 표지된 세마글루타이드(FTS)를 이용한 쥐 대상 생체분포 연구에서 긍정적인 결과를 발표했으며, DehydraTECH 기술이 GLP-1 약물 세마글루타이드의 뇌 전달을 향상시킨다고 밝혔습니다.
연구에 따르면 DehydraTECH-FTS는 뇌 생체분포가 더 높았다는 점이 확인되었고, 5mg DehydraTECH-FTS가 15mg Rybelsus® 등가물보다 뇌에서의 세마글루타이드 형광 신호 강도가 더 높았습니다. 뇌로의 개선된 전달은 특히 식욕 억제 및 메스꺼움 감소와 관련된 영역에서 GLP-1 약물의 안전성과 효능을 개선할 잠재력을 시사합니다.
이러한 발견은 Lexaria의 기술이 뇌 조직 내에서 독특한 전달 향상을 가능하게 하여 GLP-1 약물의 약력학적 성능을 개선할 수 있음을 시사합니다.
Lexaria Bioscience (Nasdaq:LEXX) a publié des résultats positifs de son étude de biodistribution chez le rongeur utilisant la semaglutide marquée de fluorescence (FTS), démontrant que la technologie DehydraTECH améliore la délivrance cérébrale du médicament GLP-1, la semaglutide.
L'étude a révélé que la DehydraTECH-FTS présentait une biodistribution cérébrale plus élevée comparée à la composition équivalente de Rybelsus®, avec la DehydraTECH-FTS à 5 mg obtenant une intensité du signal fluorescent cérébral de semaglutide plus élevée que l'équivalent de 15 mg de Rybelsus®. Cette meilleure délivrance au cerveau pourrait potentiellement améliorer la sécurité et l’efficacité des médicaments GLP-1, en particulier dans les domaines liés à la suppression de l’appétit et à la réduction des nausées.
Ces résultats suggèrent que la technologie de Lexaria pourrait permettre des améliorations de délivrance uniques dans les tissus cérébraux, soutenant potentiellement une meilleure performance pharmacodynamique des médicaments GLP-1.
Lexaria Bioscience (Nasdaq:LEXX) hat positive Ergebnisse aus ihrer fluoreszenzmarkierten Semaglutid-Studie zur Biodistribution bei Ratten bekannt gegeben, die zeigen, dass die DehydraTECH-Technologie die Gehirnabgabe des GLP-1-Arzneimittels Semaglutid verbessert.
Die Studie zeigte, dass DehydraTECH-FTS eine höhere Gehirnbiodistribution aufweist im Vergleich zur gleichwertigen Zusammensetzung von Rybelsus®, wobei DehydraTECH-FTS mit 5 mg eine höhere Intensität des fluoreszierenden Hirnsignals für Semaglutid erzielte als das 15 mg-Äquivalent von Rybelsus®. Die verbesserte Gehirnverabreichung könnte potenziell sowohl Sicherheit als auch Wirksamkeit von GLP-1-Arzneimitteln verbessern, insbesondere in Bereichen, die Appetitunterdrückung und Übelkeit betreffen.
Diese Ergebnisse deuten darauf hin, dass Lexarias Technologie einzigartige Lieferverbesserungen im Hirngewebe ermöglichen könnte und potenziell eine verbesserte pharmakodynamische Leistung von GLP-1-Arzneimitteln unterstützen könnte.
لاكسريا بايوسينس (ناسداك: LEXX) أعلنت عن نتائج إيجابية من دراستها لتوزيع الجسم في القوارض للميلفورازومن السيمياغلوتايد الم Switzerland؟ الطاقة؟ (FTS)، موضحة أن تقنية DehydraTECH تعزز توصيل GLP-1 الدوائي سيماغلوبتيد إلى الدماغ.
أظهرت الدراسة أن DehydraTECH-FTS أظهرت توزيعًا دماغيًا أعلى مقارنة بالتركيب المكافئ لـ Rybelsus®، مع أن DehydraTECH-FTS بجرعة 5 ملغ تحقق شدة إشارة فلورية دماغية لسيمياغلوتايد أعلى من مكافئ Rybelsus® بجرعة 15 ملغ. قد يحسن التوصيل المحسن إلى الدماغ السلامة والفعالية لأدوية GLP-1، لا سيما في مجالات قمع الشهية وتقليل الغثيان.
تشير هذه النتائج إلى أن تقنية ليكساريا قد تتيح تحسينات توصيل فريدة في أنسجة الدماغ، مما قد يدعم أداءً دوائيًا فيزيولوجيًا أفضل لأدوية GLP-1.
Lexaria Bioscience (纳斯达克:LEXX) 宣布了其对荧光标记的 semaglutide(FTS)在啮齿动物中的生物分布研究的积极结果,表明其 DehydraTECH 技术可提高 GLP-1 药物 semaglutide 的脑部递送。
研究显示,DehydraTECH-FTS 在脑部生物分布上更高,与等效的 Rybelsus® 成分相比,5 mg 的 DehydraTECH-FTS 在脑部 semaglutide 荧光信号强度方面高于等效的 15 mg Rybelsus®。脑部递送的改善可能在安全性与有效性方面带来潜在提升,尤其在抑制食欲和减少恶心等方面。
这些发现表明,Lexaria 的技术可能在脑组织中实现独特的递送增强,潜在地支持 GLP-1 药物的药效学表现提升。
- DehydraTECH-FTS showed superior brain biodistribution compared to Rybelsus® equivalent
- 5mg DehydraTECH-FTS achieved higher brain signal intensity than 15mg Rybelsus® equivalent, suggesting better efficiency
- Results suggest potential improvements in both safety and efficacy of GLP-1 drugs
- Findings support opportunities for industry partnerships in GLP-1 drug development
- Results are from early-stage rodent studies, requiring further research to confirm benefits
- Additional testing needed to determine optimal composition with Rybelsus® excipients
Insights
Lexaria's technology showed promising brain delivery of semaglutide in rodents, potentially improving GLP-1 drug efficacy and safety profiles.
Lexaria's DehydraTECH technology has demonstrated a significant enhancement in brain biodistribution of semaglutide in rodent studies compared to conventional formulations. Most notably, their 5mg DehydraTECH formulation achieved higher brain fluorescent signal intensity than a 15mg Rybelsus equivalent composition—suggesting a potential 3x improvement in delivery efficiency.
The findings are particularly relevant because GLP-1 drugs' mechanism of action increasingly involves brain neurochemistry. Semaglutide works by activating GLP-1 receptors in specific brain nuclei that regulate food intake, reward pathways, and energy expenditure. The enhanced brain biodistribution could explain why Lexaria has observed improvements in both safety and efficacy profiles in their previous human clinical testing.
From a pharmaceutical development perspective, this represents a potential breakthrough in addressing one of the most significant challenges with GLP-1 therapies: side effects like nausea. Studies in rodents suggest that GLP-1 analogs acting directly on the brain can suppress appetite without triggering nausea. If DehydraTECH can selectively enhance brain delivery while maintaining or improving therapeutic outcomes, it could lead to next-generation GLP-1 formulations with superior patient tolerability.
What's most intriguing is that these results were achieved with the DehydraTECH formulation without the standard Rybelsus excipients, suggesting a fundamentally different delivery mechanism. The company hypothesizes that combining their technology with existing Rybelsus excipients might yield even greater benefits, given the positive results from their earlier human pilot studies (GLP-1-H24-1 and GLP-1-H24-2).
While still early-stage research, these findings position Lexaria as a potential partner for major pharmaceutical companies looking to differentiate their GLP-1 offerings in an increasingly competitive market dominated by drugs like Ozempic, Wegovy, and Mounjaro.
Enhanced Brain Biodistribution of GLP-1 Drugs May Be Related to Improvements in Safety and Efficacy
KELOWNA, BC / ACCESS Newswire / September 19, 2025 / Lexaria Bioscience Corp. (Nasdaq:LEXX)(Nasdaq:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms, is pleased to announce successful results from its fluorescently tagged semaglutide ("FTS") rodent biodistribution study (the "Study").
The main goal of the Study was to determine whether the DehydraTECH processing of semaglutide improves its biodistribution in any significant way as compared to the conventional orally administered semaglutide formulation practice.
The most intriguing finding from the Study was that Lexaria's DehydraTECH-FTS composition, across all doses tested (asterisked below), demonstrated a predominantly higher apparent trend in brain biodistribution (evidenced as fluorescent signal intensity upon whole brain imaging) than the Rybelsus® equivalent composition and all Study controls. In fact, the 5mg DehydraTECH-FTS composition achieved higher brain semaglutide fluorescent signal intensity than the 15mg Rybelsus® equivalent composition.
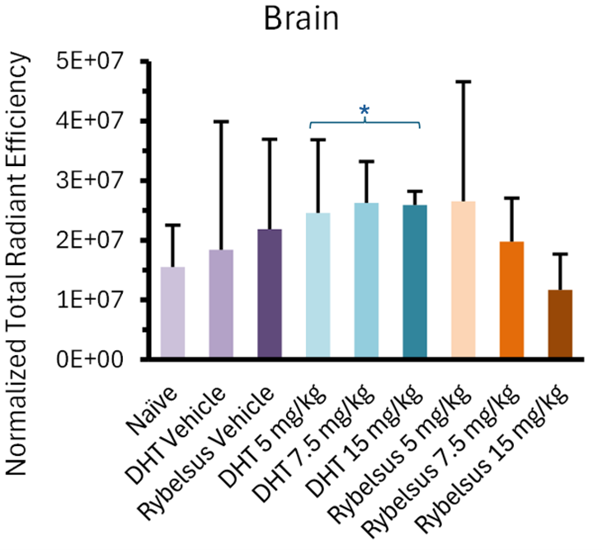
Following the whole-organ imaging, the brain was further sectioned via sagittal slices (2-3mm thickness) into two and then four pieces to better visualize the brain regions that semaglutide is known to interact with. These key regions of interest were the brainstem, known for direct semaglutide interaction; the paraventricular nucleus of the hypothalamus, involved in energy homeostasis; and the circumventricular organs, which lack a blood-brain barrier, such as the area postrema, subfornical organ, and median eminence.Upon doing so, it was noteworthy that all three DehydraTECH doses tested displayed fluorescence above that of the naïve and vehicle groups, while only the highest dosage (15mg/kg) of the Rybelsus equivalent composition surpassed the naïve and vehicle groups.
"Lexaria has repeatedly evidenced higher brain levels upon gross necropsy in rodent studies with other DehydraTECH-processed active ingredients in the past that demonstrated superior safety and efficacy over controls," said John Docherty, Lexaria President and CSO. "We are delighted to now see early evidence of this through more detailed fluorescent imaging with DehydraTECH-semaglutide which may, in turn, help to explain the performance benefits in safety and efficacy we have witnessed in our related human clinical testing to-date."
GLP-1 drug performance is increasingly understood to include or even depend upon involvement of brain neurochemistry, thus making brain biodistribution vital. Semaglutide has been shown to regulate body weight through direct and indirect activation of GLP-1 receptors ("GLP-1Rs") on several independent brain nuclei, affecting the activity of neuronal pathways involved in food intake, reward, and energy expenditure. Furthermore, studies in rodents have suggested that GLP-1 analogs can act upon brain to suppress appetite without causing nausea, which is otherwise known to be among the most common side effects of today's GLP-1 therapies. In this context, Lexaria's discovery of an apparent trend toward enhanced semaglutide brain biodistribution using its DehydraTECH technology could be impactful on the both the safety and efficacy performance of current and next generation GLP-1 drugs.
Findings from the Study suggest that the Lexaria DehydraTECH-FTS composition - absent all of the Rybelsus® equivalent composition excipients - may enable unique delivery and distribution enhancements in brain tissue possibly supporting improved pharmacodynamic performance. In addition, and perhaps to be determined through future testing, it is conceivable that complementary biodistribution benefits might be derived through utilization of a similar DehydraTECH semaglutide composition combined with the Rybelsus® excipients, recognizing that marked safety and efficacy improvements were evidenced with DehydraTECH-processed Rybelsus® over Rybelsus® alone in Lexaria's previous human pilot studies GLP-1-H24-1 and GLP-1-H24-2.
Lexaria considers these early-stage results to be highly encouraging and supportive of both additional research and of industry partnerships designed to produce the safest and most effective GLP-1 drugs on the market.
About the Study
This preclinical pilot Study was designed to evaluate the biodistribution of oral semaglutide, formulated using either DehydraTECH or a Rybelsus® equivalent composition in Sprague Dawley rats using non-invasive whole-bodyimaging and ex vivo organ analysis. The Rybelsus® equivalent composition was created by Lexaria strictly for these research purposes, with ingredients believed to be in the correct proportions used within the Rybelsus® orally-administered product sold by Novo Nordisk® today, without DehydraTECH processing. The Study involved single oral gavage dosing of the two formulations across multiple groups of rats, followed by a series of imaging time points across the different study procedures, up to 24 hours post-dosing.
The semaglutide utilized to create the FTS integrated into the test articles studied was tagged with a cyanine 7 fluorophore to enable visualization. Cyanine 7 was chosen for its excitation wavelength in the near-infrared region, allowing for deeper tissue penetration and minimal tissue autofluorescence, resulting in a lower background signal for emission measurements. Tissue collection for ex vivo imaging and further analysis, including histopathological evaluation and immunofluorescence assays, was performed to provide critical insights into the localization and distribution of semaglutide. Fluorescence signal was quantified in terms of total radiant efficiency, calculated by the in vivo imaging system imaging software, Living Image®.
FTS was tracked via fluorescent imaging detection to evidence how and where semaglutide distributed and localized following oral ingestion by the rats. Various key tissues known to expressthe GLP-1R were examined including the brain, pancreas, lung, kidney, liver, and heart. These tissues were subjected to detailed fluorescent imaging detection exams which showed more specific tissue localization patterns and concentrations.
A total of twenty-five (n=25) male Sprague Dawley rats were used in the Study, with 22 rats allocated for dosing in groups of 2-3 rats randomly assigned per formulated test article examined, and 3 additional animals designated as naïve (i.e., untreated) control. The test articles examined included vehicle formulations for both the DehydraTECH and Rybelsus® equivalent compositions, essentially serving as placebo compositions containing the respective inactive excipient ingredients but devoid of any semaglutide incorporation.
The Study was performed by an independent animal research facility specialized in pharmacokinetic evaluations. All conclusions regarding the ex vivo whole-organ imaging were made in a qualitative manner, based on visual comparisons of the images and graphs. Due to the limited size of this pilot Study, no statistical analyses or interpretations were performed.
About Lexaria Bioscience Corp. & DehydraTECH
DehydraTECH™ is Lexaria's patented drug delivery formulation and processing platform technology which improves the way a wide variety of drugs enter the bloodstream, always through oral delivery. DehydraTECH has repeatedly evidenced the ability to increase bio-absorption, reduce side effects, and deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 50 patents granted and additional patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View the original press release on ACCESS Newswire







