Lexaria Granted Strategically Important New US Patent For DehydraTECH-Nicotine Including Oral Pouch Products
- More countries are banning e-cigarettes as novel oral products gain traction
KELOWNA, BC / ACCESSWIRE / July 19, 2023 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms announces it has been granted a strategically important new patent in the oral nicotine sector by the United States Patent and Trademark Office ("USPTO").
US patent #11,700,875 Compositions and Methods For Sublingual Delivery of Nicotine includes claims for many types of nicotine, including nicotine benzoate, nicotine ditartrate, nicotine citrate, nicotine polacrilex, and many others, for use in sublingual delivery formats like oral pouches. Lexaria's superior DehydraTECH processing and sublingual compositions have now been recognized not only through exceptional scientific study results, but also by the USPTO.
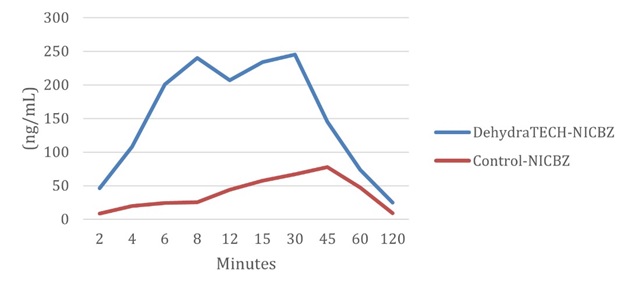
DehydraTECH-nicotine has already shown in multiple sets of animal testing that it can be delivered to the bloodstream up to 10-times to 20-times faster at up to 10-fold higher levels of nicotine into blood plasma from oral absorption than concentration matched controls. Speed of onset is of vital importance to nicotine users.
This US-awarded patent is also progressing as a patent application through other jurisdictions internationally. This US patent award and the international patent applications are supported in part by the superior pharmacokinetic blood plasma data generated by Lexaria in its animal studies conducted from 2017 through 2021. Lexaria considers the work completed by competitors to be substantially inferior when it is limited to indirect theoretical extrapolations of nicotine absorption into the bloodstream and/or subjective commentary from small numbers of people, as opposed to Lexaria's rigorous scientific examinations.
Lexaria's new patent builds nicely upon its growing patent portfolio in the oral nicotine delivery sector including the white pouch category for both sublingual and buccal tissue delivery, together with its previous patent filings specific to nicotine dating back to 2019, as well as its foundational DehydraTECH patent filings dating back to 2016.
The oral nicotine pouch category is of intense interest to Lexaria and the nicotine products industry, and its growth is due in part to its reduced risk health outcomes as noted by the Food and Drug Administration. This delivery method, in the white pouch format specifically, which avoids harmful lung outcomes experienced by smokers or vapers, involves absorption primarily through the buccal and sublingual tissues of the mouth, of purified nicotine that has been separated from most other harmful compounds in the tobacco leaf. The global market for the oral nicotine pouch category was US
Meanwhile, at least 37 countries have banned the selling of e-cigarettes, the devices used in vaping. This represents a cumulative population of over 2.3 billion people. Nicotine vaping was originally hoped to help wean people off cigarette use but has had mixed results in doing so, and vaping has also been associated with serious health risks. Nicotine vaping is becoming increasingly controversial and is severely limited in certain countries and was, for instance, recently banned in Australia and is either banned or restricted in many other countries.
Lexaria's DehydraTECH-nicotine is now patent granted for oral nicotine delivery in the U.S., Canada and Australia, and pending in numerous other countries. The white pouch category is one of the fastest growing, tobacco-free alternatives to smoking and vaping. White pouches emit no smoke, odors or vapor that can affect nearby persons and are therefore a superior personal nicotine delivery method.
The dangers of cigarette smoking are well known and result in the death of 7 million people per year. Lexaria's DehydraTECH-powered purified nicotine white pouch formulation contains no tobacco.
Nicotine Plasma Levels (ng/mL) - DehydraTECH vs. Control From Lexaria's NIC-A21-1 Study
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 35 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/768866/Lexaria-Granted-Strategically-Important-New-US-Patent-For-DehydraTECH-Nicotine-Including-Oral-Pouch-Products







