Lexaria Discovers Potential Novel Mechanism From Hypertension Study HYPER-H21-4
Lexaria Bioscience Corp. (NASDAQ: LEXX, LEXXW) has announced significant results from its clinical study HYPER-H21-4, which assessed the blood pressure-lowering effects of its DehydraTECH-processed CBD capsules. The study demonstrated a substantial and sustained reduction in blood pressure over five weeks, with no serious adverse events reported. Key findings include a 28.5% decrease in serum catestatin levels and a statistically significant drop in mean arterial pressure. These results suggest that Lexaria's CBD formulation may offer a novel therapeutic approach for managing hypertension, particularly for patients resistant to standard treatments.
- Successful achievement of primary efficacy and safety objectives in HYPER-H21-4.
- Significant blood pressure reduction observed: mean arterial pressure decreased by 4.26 mm/Hg.
- Statistically significant reduction in serum catestatin levels (28.5%) after CBD dosing.
- Potential to meet FDA guidelines for complementary anti-hypertensive therapies.
- None.
KELOWNA, BC / ACCESSWIRE / February 21, 2023 / Lexaria Bioscience Corp. (NASDAQ:LEXX)(NASDAQ:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to announce additional findings from its human clinical study HYPER-H21-4 ("the Study") demonstrating a potentially novel mechanism of action of its patented DehydraTECH-processed cannabidiol ("CBD") capsule formulation in reducing blood pressure ("BP").
The Food and Drug Administration ("FDA") has laid out clear guidelines for sponsors who seek to develop new anti-hypertensive drugs, specifically defining the need for medications that offer complementary modes of action. Lexaria believes that its latest results, detailed below and already peer-reviewed and published in the respected journal, "Biomedicine and Pharmacotherapy", may support DehydraTECH-CBD qualification within these FDA guidelines.
Lexaria previously announced that the primary efficacy and safety objectives of the Study were successfully achieved, with resting BP significantly reduced in hypertensive patients, and sustained over the full 5-weeks of dosing with zero serious adverse events being reported throughout the Study. Lexaria is aware of only a handful of other published research studies, mostly in young, healthy and normotensive volunteers, that have investigated whether a sustained decrease in resting BP is possible following multiple weeks of oral CBD dosing; none of which have been successful in achieving this.
Ongoing analyses of the Study data have revealed modulation of a circulating compound called catestatin in the patients. Catestatin is a multifunctional peptide known to have inhibitory effects on the sympathetic nervous system in the pathophysiology of hypertension.
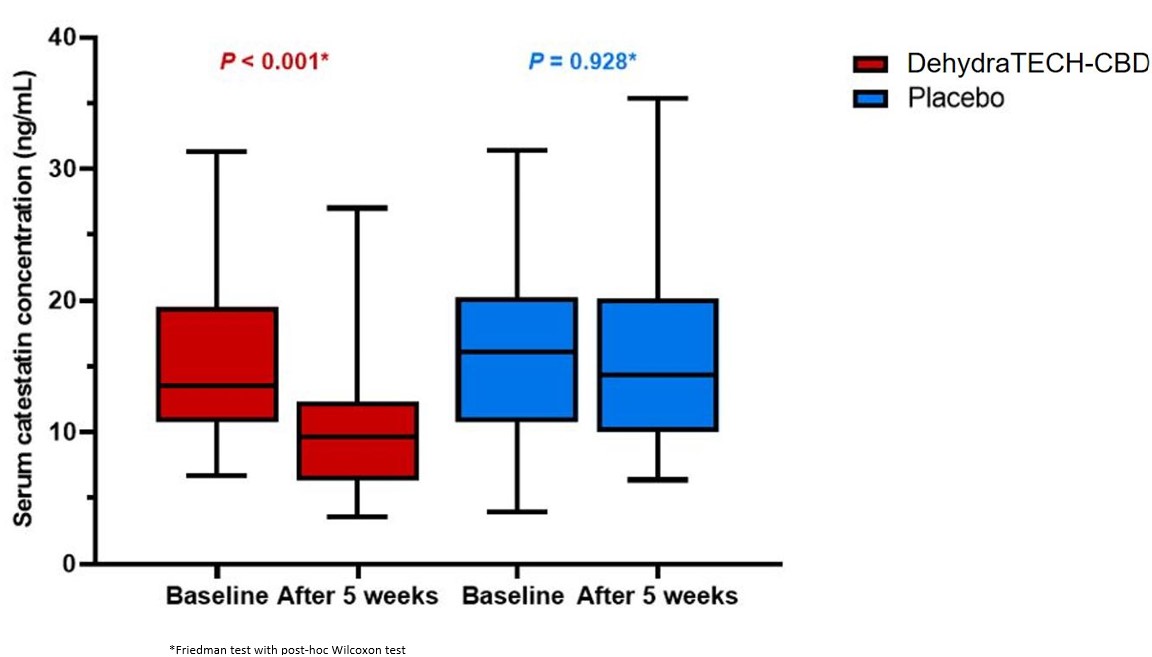
These analyses (illustrated above) revealed that administration of DehydraTECH-CBD resulted in a statistically significant reduction in average baseline serum catestatin concentrations of 13.50 ng/mL to just 9.65 ng/mL after 5 weeks of dosing, which is a large drop of
Overall, these latest results from study HYPER-H21-4 imply that the antihypertensive effects of DehydraTECH-CBD may be explained, at least in part, by its interaction with the sympatho-chromaffin system via catestatin modulation. This suggests a potentially unique mechanistic benefit upon cardiovascular regulation with DehydraTECH-CBD treatment that has not previously been demonstrated, to our knowledge, with testing of CBD for blood pressure reduction. CBD is known to have a wide array of distinct effects upon cardiovascular regulation. These include effects upon blood vessel tone, vascular inflammation, blood pressure, and cardiac contractility. The mechanisms of these observations are complex and involves interaction with receptors other than cannabinoid receptors, including actions exerted through various naturally occurring mediator compounds in the body.
There is a clear medical and market need for alternative anti-hypertensive therapies, especially in the case of patients with so-called resistant hypertension who fail to adequately control their high BP with combinations of existing medications. Of note, the decreases in BP evidenced in the Study were similar in both those hypertensive patients on standard of care BP medications and those who were untreated for their hypertension upon Study entry. Therefore, the current results indicate that Lexaria's DehydraTECH-CBD has the potential to offer complementary and additive BP reduction benefits on top of any degree of improvements the standard of care medications provided. This additive improvement of DehydraTECH-CBD as an adjunct therapy, perhaps related to its pronounced effectiveness in modulating catestatin levels, could become a significant value enhancer should it eventually enter the marketplace as an approved hypertension treatment.
Additional study endpoint analyses as described in the complete study protocol are still underway and any relevant material findings will be reported upon as these findings become available.
About Lexaria Bioscience Corp.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream through oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 28 patents granted and many patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
Investor Contact:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/740061/Lexaria-Discovers-Potential-Novel-Mechanism-From-Hypertension-Study-HYPER-H21-4
FAQ
What were the findings of Lexaria's HYPER-H21-4 study?
How much did blood pressure lower in the HYPER-H21-4 study?
What is the significance of catestatin in Lexaria's study?
What is Lexaria's stock symbol?







