Innovent Dosed First Participant in Phase 3 Clinical Study (GLORY-2) of Mazdutide (IBI362) Higher Dose 9 mg in Chinese Adults with Obesity
- Successful dosing of the first participant in Phase 3 clinical trial of mazdutide
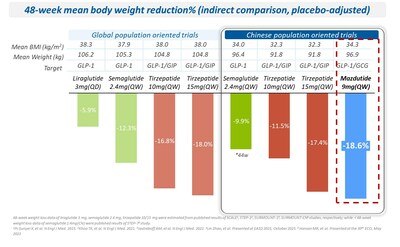
- Promising results from Phase 2 study with 18.6% placebo-adjusted mean percent reduction in body weight and multiple metabolic benefits
- None.
GLORY-2 plans to enroll 450 subjects and randomize to receive mazdutide 9 mg treatment or placebo. The primary endpoints include the percentage change in body weight from baseline to week 60 and the proportion of subjects with
A Phase 2 study conducted in Chinese people with obesity with a BMI ≥ 30 kg/m2 (NCT04904913) showed that after 48 weeks of treatment, mazdutide 9mg achieved
Professor Linong Ji, the principal investigator of the study, Peking University People's Hospital, stated, "Accumulated evidence showed that Chinese people generally have higher percentages of body fat and higher rates of cardiovascular risk factors and all-cause mortality than western population at given BMI levels, therefore overweight and obesity in adults in
Dr. Lei Qian, Vice President of Clinical Development at Innovent, stated, "Different dosage regimens of mazdutide are suitable for different groups of people with obesity. Mazdutide 9 mg is specifically developed to meet the weight loss needs of people with moderate to severe obesity with a BMI above 30 kg/m2 in
About Obesity
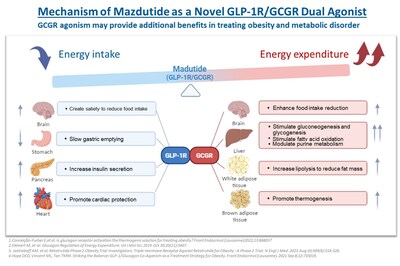
About Mazdutide (IBI362)
Innovent entered into an exclusive license agreement with Eli Lilly and Company (Lilly) for the development and potential commercialization of OXM3 (also known as mazdutide), a GLP-1R and GCGR dual agonist, in
About Innovent
Inspired by the spirit of "Start with Integrity, Succeed through Action," Innovent's mission is to discover and develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people. Established in 2011, Innovent is committed to discovering and developing, manufacturing and commercializing high-quality innovative medicines for the treatment of oncology, autoimmune, cardiovascular and metabolic, and ophthalmology diseases to enhance the quality of the patients' lives. Innovent has 10 products in the market, including TYVYT® (Sintilimab Injection), BYVASDA® (Bevacizumab Injection), SULINNO® (Adalimumab Injection), HALPRYZA® (Rituximab Injection), Pemazyre® (Pemigatinib Oral Inhibitor), olverembatinib, Cyramza® (Ramucirumab Injection), Retsevmo® (Selpercatinib Capsules ), FUCASO® (Equecabtagene Autoleucel Injection) and SINTBILO® (Tafolecimab Injection). Additionally, we have 2 NDA under NMPA review, 5 assets in Phase III or pivotal clinical trials, and 19 more molecules in early clinical stage.
Innovent has also entered into 30 strategic collaborations with Eli Lilly, Roche, Sanofi, Adimab, Incyte, MD Anderson Cancer Center and other international partners. We strive to work with many collaborators to help advance the biopharmaceutical industry, improve drug availability and enhance the quality of the patients' lives.
Note:
TYVYT® (sintilimab injection) is not an approved product in
BYVASDA® (bevacizumab biosimilar injection), SULINNO®, and HALPRYZA® (rituximab biosimilar injection) are not approved products in
TYVYT® (sintilimab injection, Innovent)
BYVASDA® (bevacizumab biosimilar injection, Innovent)
HALPRYZA® (rituximab biosimilar injection, Innovent)
SULINNO® (adalimumab biosimilar injection, Innovent)
Pemazyre® (pemigatinib oral inhibitor, Incyte Corporation). Pemazyre® was discovered by Incyte Corporation and licensed to Innovent for development and commercialization in Mainland China,
CYRAMZA® (ramucirumab, Eli Lilly). Cyramza® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Retsevmo® (selpercatinib, Eli Lilly). Retsevmo® was discovered by Eli Lilly and licensed to Innovent for commercialization in Mainland China.
Disclaimer: Innovent does not recommend any off-label usage.
Forward-looking statement
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent Biologics ("Innovent"), are intended to identify certain of such forward-looking statements. The Company does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of the Company with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond the Company's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, the Company's competitive environment and political, economic, legal and social conditions.
The Company, the Directors and the employees of the Company assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialise or turn out to be incorrect.
[i] Body-mass index and obesity in urban and rural |
[ii] https://www.innoventbio.com/InvestorsAndMedia/PressReleaseDetail?key=407 |
[iii] Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in |
[iv] Institute for Health Metrics and Evaluation. Global Health Data Exchange. GBD results tool. http://ghdx.healthdata.org/gbd-resultstool (accessed Jan 10, 2021). |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-dosed-first-participant-in-phase-3-clinical-study-glory-2-of-mazdutide-ibi362-higher-dose-9-mg-in-chinese-adults-with-obesity-302024183.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/innovent-dosed-first-participant-in-phase-3-clinical-study-glory-2-of-mazdutide-ibi362-higher-dose-9-mg-in-chinese-adults-with-obesity-302024183.html
SOURCE Innovent Biologics