Immuneering Provides Positive Update on Phase 2a Arm Studying IMM-1-104 in Combination with Modified FOLFIRINOX for First-Line Pancreatic Cancer
Immuneering (Nasdaq: IMRX) has announced positive updates from its Phase 2a clinical trial evaluating IMM-1-104 in combination with modified FOLFIRINOX (mFFX) for first-line pancreatic cancer. The trial reported two new partial responses (PRs), resulting in an overall response rate (ORR) of 50%, surpassing the historic benchmark of 32% for FOLFIRINOX alone. As of January 6, 2025, six patients were evaluable, with three achieving partial responses.
The combination therapy was generally well tolerated. Immuneering raised nearly $14 million in net proceeds through its ATM facility, enhancing its cash balance. The company plans to provide further Phase 2a data in the second quarter of 2025 and to initiate new trial arms combining IMM-1-104 with a BRAF inhibitor and a checkpoint inhibitor. Additionally, planning for a pivotal trial of IMM-1-104 with modified gemcitabine/nab-paclitaxel is underway.
Immuneering (Nasdaq: IMRX) ha annunciato aggiornamenti positivi dal suo trial clinico di fase 2a che valuta IMM-1-104 in combinazione con FOLFIRINOX modificato (mFFX) per il cancro pancreatico di prima linea. Il trial ha riportato due nuove risposte parziali (PR), portando a un tasso di risposta complessivo (ORR) del 50%, superando il benchmark storico del 32% per FOLFIRINOX da solo. A partire dal 6 gennaio 2025, sei pazienti erano valutabili, con tre che hanno raggiunto risposte parziali.
La terapia combinata è stata generalmente ben tollerata. Immuneering ha raccolto quasi $14 milioni in proventi netti attraverso la propria struttura ATM, migliorando il proprio bilancio di cassa. L'azienda prevede di fornire ulteriori dati di fase 2a nel secondo trimestre del 2025 e di avviare nuovi bracci trial combinando IMM-1-104 con un inibitore BRAF e un inibitore del checkpoint. Inoltre, è in corso la pianificazione di un trial decisivo per IMM-1-104 con gemcitabina modificata/nab-paclitaxel.
Immuneering (Nasdaq: IMRX) ha anunciado actualizaciones positivas de su ensayo clínico de fase 2a que evalúa IMM-1-104 en combinación con FOLFIRINOX modificado (mFFX) para el cáncer de páncreas en primera línea. El ensayo reportó dos nuevas respuestas parciales (PR), resultando en una tasa de respuesta general (ORR) del 50%, superando el punto de referencia histórico del 32% para FOLFIRINOX solo. A partir del 6 de enero de 2025, seis pacientes fueron evaluables, con tres logrando respuestas parciales.
La terapia combinada fue generalmente bien tolerada. Immuneering recaudó casi $14 millones en ingresos netos a través de su instalación ATM, mejorando su saldo de efectivo. La compañía planea proporcionar más datos de fase 2a en el segundo trimestre de 2025 y comenzar nuevos brazos de ensayo combinando IMM-1-104 con un inhibidor de BRAF y un inhibidor de checkpoint. Además, se está planificando un ensayo pivotal de IMM-1-104 con gemcitabina/nab-paclitaxel modificado.
Immuneering (Nasdaq: IMRX)는 1상 2a 임상 시험 결과 IMM-1-104를 변형된 FOLFIRINOX (mFFX)와 병용하여 췌장암 1차 치료제로 평가한 긍정적인 업데이트를 발표했습니다. 이 시험은 두 가지 새로운 부분 반응(PR)을 보고했으며, 이로 인해 전체 반응률(ORR)은 50%로, 단독 FOLFIRINOX의 역사적 기준인 32%를 초과했습니다. 2025년 1월 6일 기준으로 평가 가능한 환자는 6명이며, 그 중 3명이 부분 반응을 보였습니다.
복합 요법은 일반적으로 잘 견뎌졌습니다. Immuneering은 ATM 시설을 통해 거의 1,400만 달러의 순수익을 올려 현금 잔고를 강화했습니다. 회사는 2025년 2분기에 추가적인 2a상 데이터를 제공할 계획이며, IMM-1-104와 BRAF 억제제 및 체크포인트 억제제를 결합한 새로운 시험 분기를 시작할 예정입니다. 또한, 변형된 겐시타빈/nab-paclitaxel과 함께하는 IMM-1-104의 결정적 시험에 대한 계획이 진행 중입니다.
Immuneering (Nasdaq: IMRX) a annoncé des mises à jour positives de son essai clinique de phase 2a évaluant IMM-1-104 en combinaison avec FOLFIRINOX modifié (mFFX) pour le cancer du pancréas en première ligne. L'essai a signalé deux nouvelles réponses partielles (PR), entraînant un taux de réponse global (ORR) de 50%, dépassant l'étalon historique de 32% pour le FOLFIRINOX seul. Au 6 janvier 2025, six patients étaient évaluables, dont trois ont obtenu des réponses partielles.
La thérapie combinée a été généralement bien tolérée. Immuneering a levé près de 14 millions de dollars de revenus nets grâce à sa structure ATM, renforçant ainsi sa trésorerie. L'entreprise prévoit de fournir d'autres données de phase 2a au deuxième trimestre de 2025 et de lancer de nouveaux bras d'essai combinant IMM-1-104 avec un inhibiteur de BRAF et un inhibiteur de point de contrôle. De plus, la planification d'un essai pivot de IMM-1-104 avec la gemcitabine modifiée/nab-paclitaxel est en cours.
Immuneering (Nasdaq: IMRX) hat positive Updates aus seiner Phase 2a klinischen Studie bekannt gegeben, die IMM-1-104 in Kombination mit modifiziertem FOLFIRINOX (mFFX) zur Erstlinientherapie von Bauchspeicheldrüsenkrebs evaluiert. Die Studie berichtete von zwei neuen partiellen Antworte (PRs), was zu einer Gesamtansprechrate (ORR) von 50% führte, die den historischen Benchmark von 32% für FOLFIRINOX alleine übertrifft. Zum 6. Januar 2025 waren sechs Patienten evaluiert, von denen drei partielle Antworten erzielten.
Die Kombinationstherapie wurde im Allgemeinen gut vertragen. Immuneering hat fast 14 Millionen Dollar an Nettoerlösen durch seine ATM-Anlage gesammelt, was die Barreserven erhöht. Das Unternehmen plant, im zweiten Quartal 2025 weitere Phase 2a-Daten bereitzustellen und neue Studienarme zu starten, die IMM-1-104 mit einem BRAF-Inhibitor und einem Checkpoint-Inhibitor kombinieren. Zudem wird ein pivotaler Test von IMM-1-104 mit modifiziertem Gemcitabin/nab-Paclitaxel geplant.
- Two new partial responses reported in Phase 2a trial.
- Overall response rate (ORR) of 50%, surpassing the 32% benchmark for FOLFIRINOX alone.
- Combination therapy was generally well tolerated.
- Raised nearly $14 million in net proceeds, strengthening cash balance.
- Further Phase 2a data expected in Q2 2025.
- None.
Insights
The latest update from Immuneering's Phase 2a trial reveals compelling efficacy signals for IMM-1-104 in combination with modified FOLFIRINOX for first-line pancreatic cancer treatment. The 50% overall response rate (3/6 patients) significantly outperforms the historical benchmark of 32% for FOLFIRINOX monotherapy. This is particularly noteworthy in pancreatic cancer, where treatment advances have been notoriously difficult to achieve.
The combination's tolerability profile and the fact that 4 patients remain on treatment suggests a manageable safety profile - a important factor for combination therapies in pancreatic cancer where patients often struggle with treatment-related toxicities. The planned expansion into BRAF inhibitor and checkpoint inhibitor combinations indicates confidence in IMM-1-104's potential as a versatile combination partner.
The
The company's dual-track approach - advancing both clinical development and securing additional funding - positions them well for upcoming milestones. The planned expansion into new combination studies and preparation for pivotal trials suggests accelerated development timelines, which could catalyze significant value creation. However, investors should note that as a small-cap biotech, the company will likely require additional funding rounds to support late-stage development.
The rapid progression of IMM-1-104's development program is noteworthy. Having two parallel first-line pancreatic cancer combination arms showing comparable response rates strengthens the drug's potential positioning. The planned expansion into BRAF inhibitor and checkpoint inhibitor combinations in 2025 suggests a comprehensive development strategy targeting multiple therapeutic approaches.
The Q2 2025 data update will be crucial, as it will provide more mature data with potentially longer follow-up and additional patients. The preparation for a pivotal trial with modified gemcitabine/nab-paclitaxel combination indicates confidence in the program's trajectory. For context, successful development of a well-tolerated MEK inhibitor that can be effectively combined with standard therapies would address a significant unmet need in oncology.
- Two new partial responses (PRs) reported in Phase 2a arm studying IMM-1-104 in combination with modified FOLFIRINOX (mFFX) in first-line pancreatic cancer -
- Overall Response Rate (ORR) of
- Nearly
CAMBRIDGE, Mass., Jan. 13, 2025 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients, today announced a positive update from its Phase 2a arm studying IMM-1-104 in combination with modified FOLFIRINOX (mFFX) in first-line pancreatic cancer, and provided a corporate update.
“We are thrilled to report two more responses in our Phase 2a arm studying IMM-1-104 in combination with modified FOLFIRINOX in first-line pancreatic cancer,” said Ben Zeskind, Ph.D., CEO of Immuneering. “The response rates emerging from both of our Phase 2a combination arms in first-line pancreatic cancer are comparable to one another, and speak not only to IMM-1-104’s potential to drive a new standard of care in pancreatic cancer, but also its potential as a first-of-its-kind, well-tolerated MEK inhibitor that could be safely used in a variety of combinations to drive better outcomes for patients across a range of indications.”
Zeskind continued: “Building on our positive January 7, 2025 data update, we strengthened our cash balance with nearly
Data Update from Phase 2a Arm Evaluating IMM-1-104 with Modified FOLFIRINOX in First Line Pancreatic Cancer as of January 6, 2025
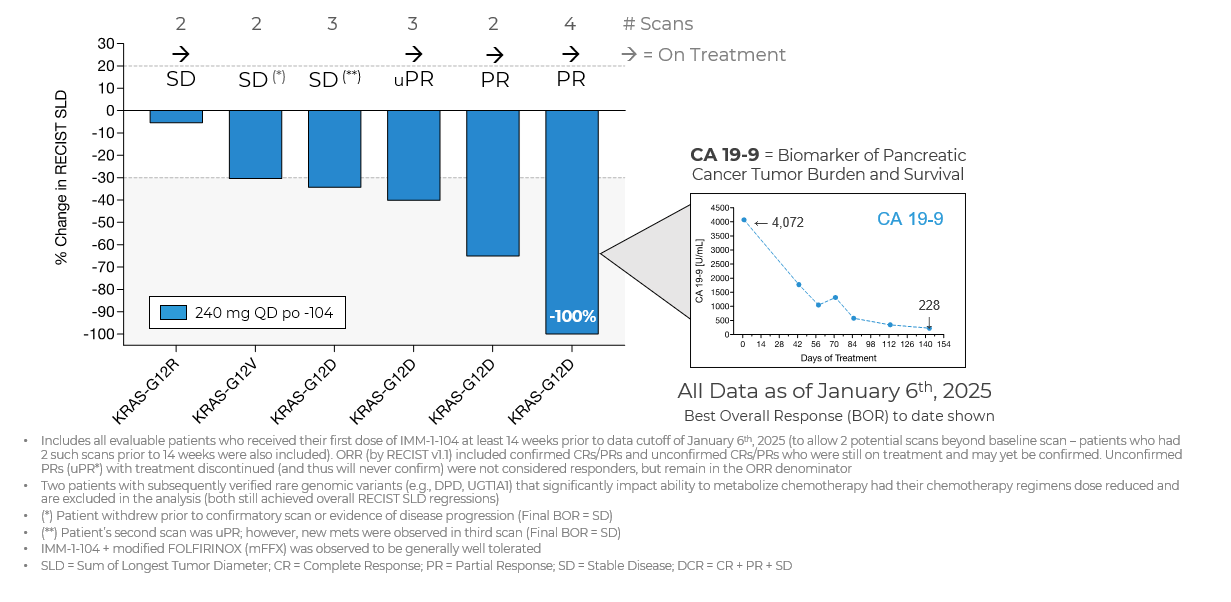
Source: Immuneering Corporation
- Since the Company’s prior update on January 7, 2025 (which used a data cutoff date of December 5, 2024), two new partial responses (PRs) have been reported.
- As of January 6, 2025, there were six evaluable patients in the Phase 2a arm evaluating IMM-1-104 with modified FOLFIRINOX in first-line pancreatic cancer; three patients achieved partial responses (one unconfirmed) for an overall response rate of
50% (3/6). Four patients remain on treatment. The historic benchmark ORR is32% for FOLFIRINOX alone. - The combination of IMM-1-104 plus modified FOLFIRINOX (mFFX) was observed to be generally well tolerated.
- The Company is currently evaluating the 320 mg QD dose of IMM-1-104 in combination with modified FOLFIRINOX.
Additionally, Immuneering today announced that it raised net proceeds of
Near-Term Milestone Expectations
IMM-1-104
- Further IMM-1-104 Phase 2a data expected in the second quarter of 2025
- Initiation of Phase 2a arms of IMM-1-104 in combination with a BRAF inhibitor, as well as IMM-1-104 in combination with a checkpoint inhibitor, planned for 2025
About Immuneering Corporation
Immuneering is a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients. The Company’s lead product candidate, IMM-1-104, is an oral, once-daily deep cyclic inhibitor of MEK designed to improve tolerability and expand indications to include RAS-driven tumors such as most pancreatic cancers. IMM-1-104 is currently in a Phase 1/2a trial in patients with advanced solid tumors including pancreatic cancer. IMM-6-415 is an oral, twice-daily deep cyclic inhibitor of MEK currently in a Phase 1/2a trial in patients with advanced solid tumors harboring RAS or RAF mutations. The company’s development pipeline also includes several early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: Immuneering’s plans to develop, manufacture and commercialize its product candidates; the treatment potential of IMM-1-104, alone or in combination with other agents, including chemotherapy, checkpoint inhibitors and BRAF inhibitors; the plans and objectives of Company management for future operations, including with respect to the planning and execution of additional IMM-1-104 combination trials and potential pivotal trial of IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel; and the timing for release of additional results from the Phase 2a portion of the trial for IMM-1-104.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding and ability to continue as a going concern; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the period ended September 30, 2024, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Gina Nugent
gina@nugentcommunications.com
Investor Contact:
Laurence Watts
619-916-7620
laurence@newstreetir.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/fabe23d6-4839-489f-93d8-7d65f946f5ee








