Immuneering Announces Positive Data Update from Three Pancreatic Cancer Arms of Ongoing Phase 2a Trial of IMM-1-104; Plans to Expand Trial with Additional Arms
Immuneering (NASDAQ: IMRX) reported positive data from three pancreatic cancer arms of its Phase 2a trial for IMM-1-104. The drug showed a 43% overall response rate (ORR) and 86% disease control rate (DCR) when combined with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer patients.
Initial data for IMM-1-104 with modified FOLFIRINOX in first-line pancreatic cancer showed tumor shrinkage in all evaluable patients, including a 100% reduction. In second-line pancreatic cancer monotherapy, the drug demonstrated a 67% reduction in one patient.
The company plans to expand the trial with three additional combination arms in 2025, including combinations with BRAF inhibitor in melanoma and immune checkpoint inhibitors in both melanoma and NSCLC. Further Phase 2a data is expected in Q2 2025.
Immuneering (NASDAQ: IMRX) ha riportato dati positivi da tre bracci di cancro pancreatico del suo trial di Fase 2a per IMM-1-104. Il farmaco ha mostrato un tasso di risposta globale (ORR) del 43% e un tasso di controllo della malattia (DCR) dell'86% quando combinato con gemcitabina/nab-paclitaxel modificato nei pazienti con cancro pancreatico in prima linea.
I dati iniziali per IMM-1-104 con FOLFIRINOX modificato in cancro pancreatico in prima linea hanno mostrato una riduzione dei tumori in tutti i pazienti valutabili, incluso un 100% di riduzione. Nella monoterapia di cancro pancreatico di seconda linea, il farmaco ha dimostrato una riduzione del 67% in un paziente.
La società prevede di espandere lo studio con tre ulteriori bracci di combinazione nel 2025, comprese le combinazioni con inibitori di BRAF nel melanoma e inibitori del checkpoint immunitario sia nel melanoma che nel NSCLC. Ulteriori dati di Fase 2a sono attesi nel Q2 2025.
Immuneering (NASDAQ: IMRX) informó datos positivos de tres brazos de cáncer de páncreas de su ensayo de Fase 2a para IMM-1-104. El fármaco mostró una tasa de respuesta global (ORR) del 43% y una tasa de control de la enfermedad (DCR) del 86% cuando se combinó con gemcitabina/nab-paclitaxel modificado en pacientes de cáncer de páncreas en primera línea.
Los datos iniciales para IMM-1-104 con FOLFIRINOX modificado en cáncer de páncreas en primera línea mostraron reducción del tumor en todos los pacientes evaluables, incluida una reducción del 100%. En la monoterapia de cáncer de páncreas en segunda línea, el fármaco demostró una reducción del 67% en un paciente.
La compañía planea expandir el ensayo con tres brazos de combinación adicionales en 2025, incluyendo combinaciones con inhibidores de BRAF en melanoma y con inhibidores de puntos de control inmunitario tanto en melanoma como en NSCLC. Se esperan más datos de Fase 2a en el segundo trimestre de 2025.
Immuneering (NASDAQ: IMRX)는 IMM-1-104의 2상 시험에서 췌장암 관련 세 가지 그룹의 긍정적인 데이터를 보고했습니다. 이 약물은 첫 번째 치료에 췌장암 환자에게 수정된 젬시타빈/nab-파클리탁셀과 함께 사용할 때 43%의 전체 반응률 (ORR)과 86%의 질병 조절률 (DCR)을 나타냈습니다.
1 차 췌장암에서 수정된 FOLFIRINOX와 함께한 IMM-1-104의 초기 데이터는 평가 가능한 모든 환자에서 종양 축소를 보였고, 그 중 한 환자는 100%의 감소를 보였습니다. 2차 치료의 췌장암 단독 요법에서 이 약물은 한 환자에서 67% 감소를 입증했습니다.
회사는 2025년에 BRAF 억제제와 악성 흑색종에 대한 조합, 그리고 면역 체크포인트 억제제와 NSCLC에 대한 조합을 포함해 3개의 추가 조합 그룹으로 시험 확대를 계획하고 있습니다. 추가적인 2상 데이터는 2025년 2분기에 기대됩니다.
Immuneering (NASDAQ: IMRX) a rapporté des données positives provenant de trois bras de cancer du pancréas de son essai de Phase 2a pour IMM-1-104. Le médicament a montré un taux de réponse global (ORR) de 43% et un taux de contrôle de la maladie (DCR) de 86% lorsqu'il est combiné avec de la gemcitabine/nab-paclitaxel modifiée chez des patients atteints de cancer du pancréas en première ligne.
Les données initiales pour IMM-1-104 avec FOLFIRINOX modifié en première ligne de cancer du pancréas ont montré une réduction de la tumeur chez tous les patients évaluables, y compris une réduction de 100%. En monothérapie pour le cancer du pancréas de deuxième ligne, le médicament a démontré une réduction de 67% chez un patient.
La société prévoit d'élargir l'essai avec trois bras de combinaison supplémentaires en 2025, y compris des combinaisons avec des inhibiteurs de BRAF dans le mélanome et des inhibiteurs de points de contrôle immunitaire tant dans le mélanome que dans le NSCLC. D'autres données de la phase 2a sont attendues au deuxième trimestre 2025.
Immuneering (NASDAQ: IMRX) berichtete über positive Daten aus drei Armen der Pankreaskrebsstudie der Phase 2a für IMM-1-104. Das Medikament zeigte eine Gesamtansprechrate (ORR) von 43% und eine Krankheitskontrollrate (DCR) von 86%, als es mit modifiziertem Gemcitabin/nab-Paclitaxel bei Pankreaskrebspatienten der ersten Linie kombiniert wurde.
Erste Daten für IMM-1-104 mit modifiziertem FOLFIRINOX in der ersten Reihe bei Pankreaskrebs zeigten bei allen auswertbaren Patienten Tumorreduzierung, einschließlich einer 100%igen Reduktion. In der Monotherapie bei sekundärem Pankreaskrebs zeigte das Medikament eine Reduktion von 67% bei einem Patienten.
Das Unternehmen plant, die Studie 2025 mit drei weiteren Kombinationsarmen zu erweitern, die Kombinationen mit BRAF-Hemmern beim Melanom und Immun-Checkpoint-Hemmern sowohl beim Melanom als auch beim NSCLC umfassen. Weitere Phase-2a-Daten werden im 2. Quartal 2025 erwartet.
- 43% ORR and 86% DCR in first-line pancreatic cancer, significantly higher than benchmark of 23% ORR and 48% DCR
- 100% reduction (PR) observed in combination with FOLFIRINOX in first-line pancreatic cancer
- 67% tumor reduction achieved in second-line pancreatic cancer monotherapy
- Highly differentiated safety profile with no Grade 3 or 4 adverse events in monotherapy
- FDA Fast Track designation received for first- and second-line pancreatic cancer
- Small patient sample size in current data (7 patients in gemcitabine/nab-paclitaxel arm)
- Additional trial arms will require more time and resources to complete
Insights
The Phase 2a trial data for IMM-1-104 represents a significant breakthrough in pancreatic cancer treatment. The 43% overall response rate (ORR) and 86% disease control rate (DCR) in combination with modified gemcitabine/nab-paclitaxel substantially outperform historical benchmarks of 23% ORR and 48% DCR. The 100% tumor reduction in the FOLFIRINOX combination arm and 67% reduction in monotherapy demonstrate remarkable efficacy across multiple treatment settings.
The tolerability profile is particularly noteworthy - the absence of Grade 3/4 adverse events in the monotherapy arm suggests a potential paradigm shift in MEK inhibition. This could enable broader combination strategies and longer treatment durations, critical factors in improving patient outcomes.
The commercial implications are substantial. The current MEK inhibitor market generates
With three new combination arms planned, including melanoma and NSCLC applications, Immuneering is strategically positioning IMM-1-104 across multiple high-value oncology indications. This could substantially expand the drug's commercial potential beyond the current MEK inhibitor market.
For a company with a market cap of just
The robust efficacy data, combined with the superior safety profile, positions IMM-1-104 as a potential best-in-class therapy. This could attract partnership interest from larger pharmaceutical companies, particularly given the broad applicability across multiple cancer types and the favorable combination potential with existing therapies.
- Updated data for IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel (mGnP) in first-line pancreatic cancer patients show favorable overall response rate (ORR =
- Favorable initial data for IMM-1-104 in combination with modified FOLFIRINOX (mFFX) in first-line pancreatic cancer patients show target lesion shrinkage in all evaluable patients, including a
- Encouraging initial data for IMM-1-104 monotherapy in second-line pancreatic cancer, including a
- Continued highly differentiated tolerability profile observed for IMM-1-104;
approved MEK inhibitors currently drive ~
- Further IMM-1-104 Phase 2a data expected in 2Q’25; additional combination arms planned to be initiated in 2025 -
- Company to hold webcast today at 8.30 am ET /5:30 am PT -
CAMBRIDGE, Mass., Jan. 07, 2025 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients, today announced a positive data update from three pancreatic cancer arms of its ongoing Phase 2a trial of lead program IMM-1-104, as well as plans to expand the Phase 2a trial to include three additional combination arms. While approved MEK inhibitors mainly benefit a subset of patients with BRAF-driven tumors, IMM-1-104 was designed to improve tolerability and expand indications to include RAS-mutated tumors such as those found in most pancreatic cancers.
“We are excited to report an updated ORR of
Dr. Zeskind continued: “Today we are also sharing initial data from IMM-1-104 in combination with modified FOLFIRINOX in first-line pancreatic cancer patients. We observed target lesion shrinkage across all evaluable patients, including a
Dr. Zeskind concluded: “Importantly, we continue to observe a highly differentiated safety profile for IMM-1-104, which we designed to be better tolerated and more active than existing approved MEK inhibitors already driving annual net sales of ~
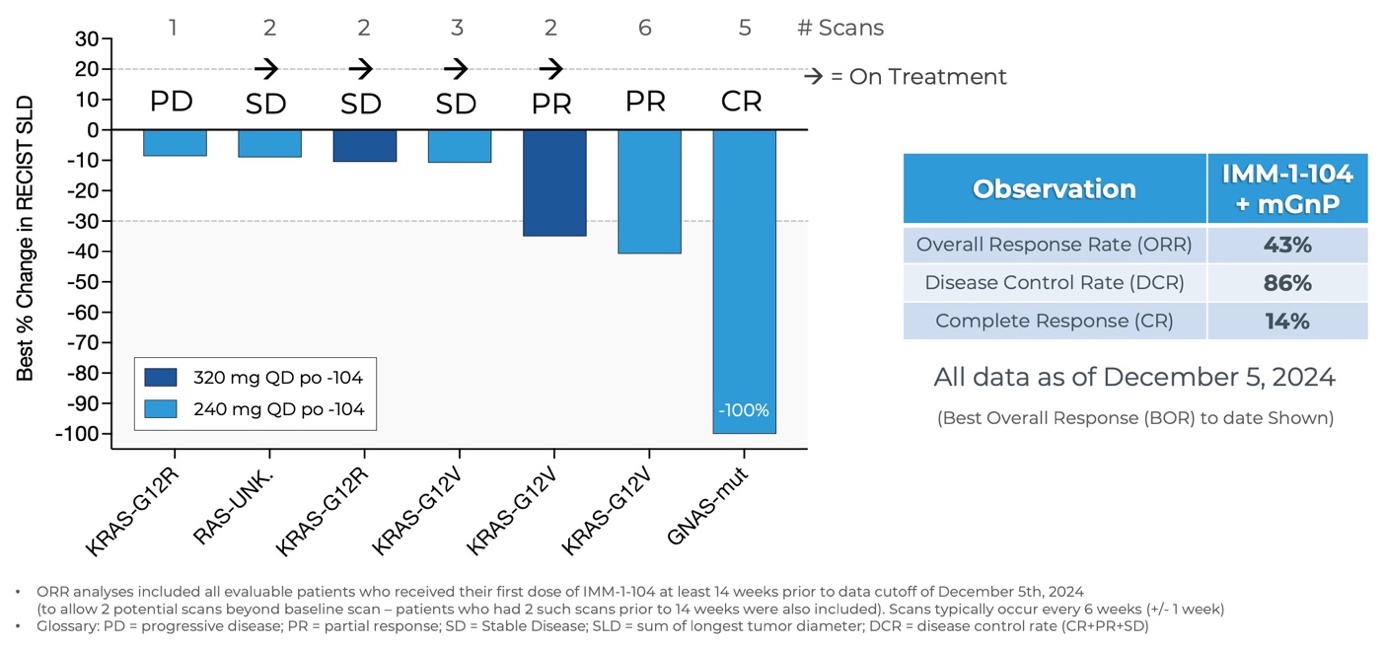
Updated Data from Phase 2a Arm Evaluating IMM-1-104 with Modified Gemcitabine/nab-Paclitaxel in First Line Pancreatic Cancer as of December 5, 2024
Source: Immuneering Corporation
- As of December 5, 2024, three patients in the Phase 2a arm evaluating IMM-1-104 with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer achieved complete or partial responses for an overall response rate of
43% (3/7) and disease control rate of86% (6/7). Four patients remain on treatment. - Benchmarks for gemcitabine/nab-paclitaxel alone in first-line pancreatic cancer patients were established by the Phase 3 MPACT study, which included 1 Complete Response (CR) out of 431 patients, a
23% Overall Response Rate, and a48% Disease Control Rate1. Benchmarks for modified (m) Gemcitabine/nab-Paclitaxel, the less intensive regimen utilized in the IMM-1-104 Phase 2 combination arm, include an18.6% ORR2. - A favorable tolerability profile was observed for IMM-1-104 in combination with modified Gemcitabine/nab-Paclitaxel.
[1] Von Hoff, et al. N Engl J Med 2013;369:1691-1703, [2] Ahn DH, et al. Therapeutic Advances in Medical Oncology. 2017;9(2):75-82
“Immuneering’s Phase 2a data in first-line pancreatic cancer are very promising,” said Tanios Bekaii-Saab, M.D., Leader of the Gastrointestinal Cancer Disease Group for the Mayo Clinic Cancer Center enterprise-wide and Medical Oncology consultant in Mayo Clinic in Phoenix, Arizona. “If current trends continue, the combination of IMM-1-104 with modified gemcitabine/nab-paclitaxel may provide improved efficacy and tolerability versus gemcitabine/nab-paclitaxel in the first-line pancreatic cancer setting, where patients continue to urgently need better options. In addition, having a MEK inhibitor that appears to be as well-tolerated as IMM-1-104 may provide new opportunities for patients with different types of cancer.”
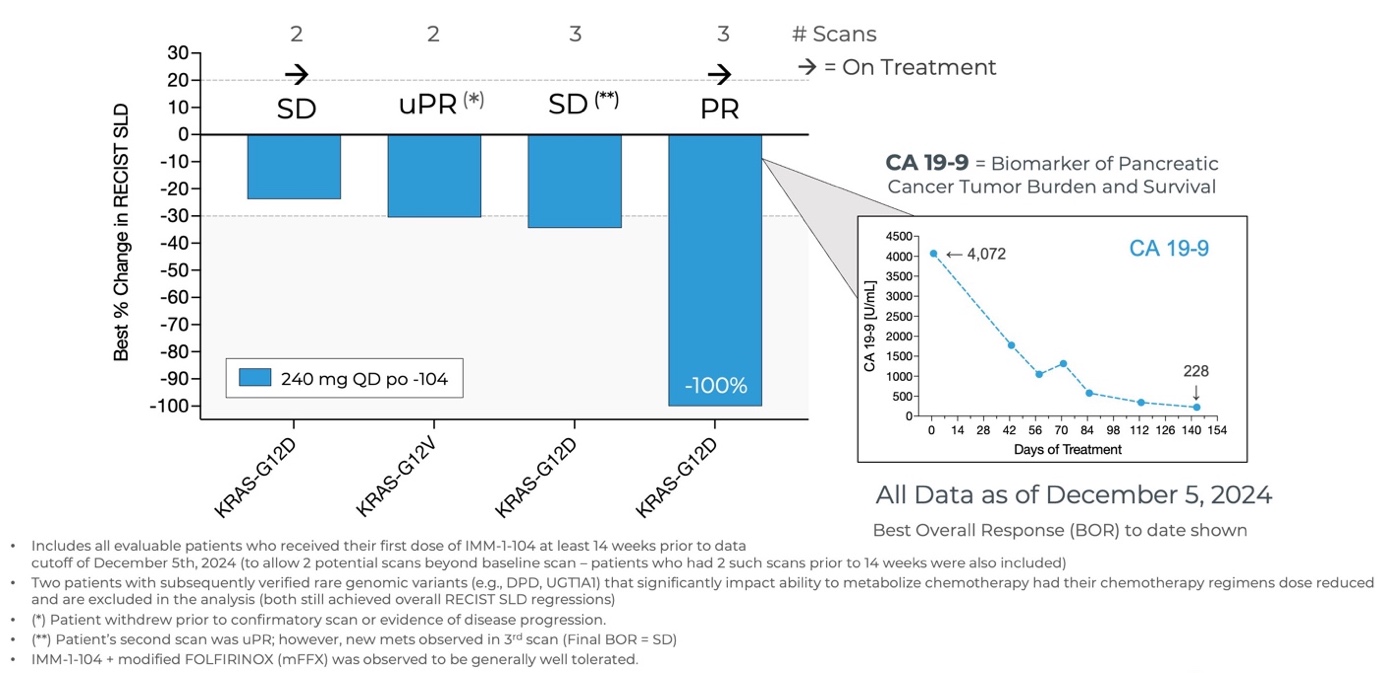
Initial Data from Phase 2a Arm Evaluating IMM-1-104 with Modified FOLFIRINOX in First Line Pancreatic Cancer as of December 5, 2024
Source: Immuneering Corporation
- As of December 5, 2024, all evaluable patients (n=4) experienced target tumor shrinkage and disease control, with one patient achieving a
100% reduction (PR). - The combination of IMM-1-104 plus modified FOLFIRINOX (mFFX) was observed to be generally well tolerated.
- The Company is currently evaluating the 320 mg QD dose of IMM-1-104 in combination with modified FOLFIRINOX.
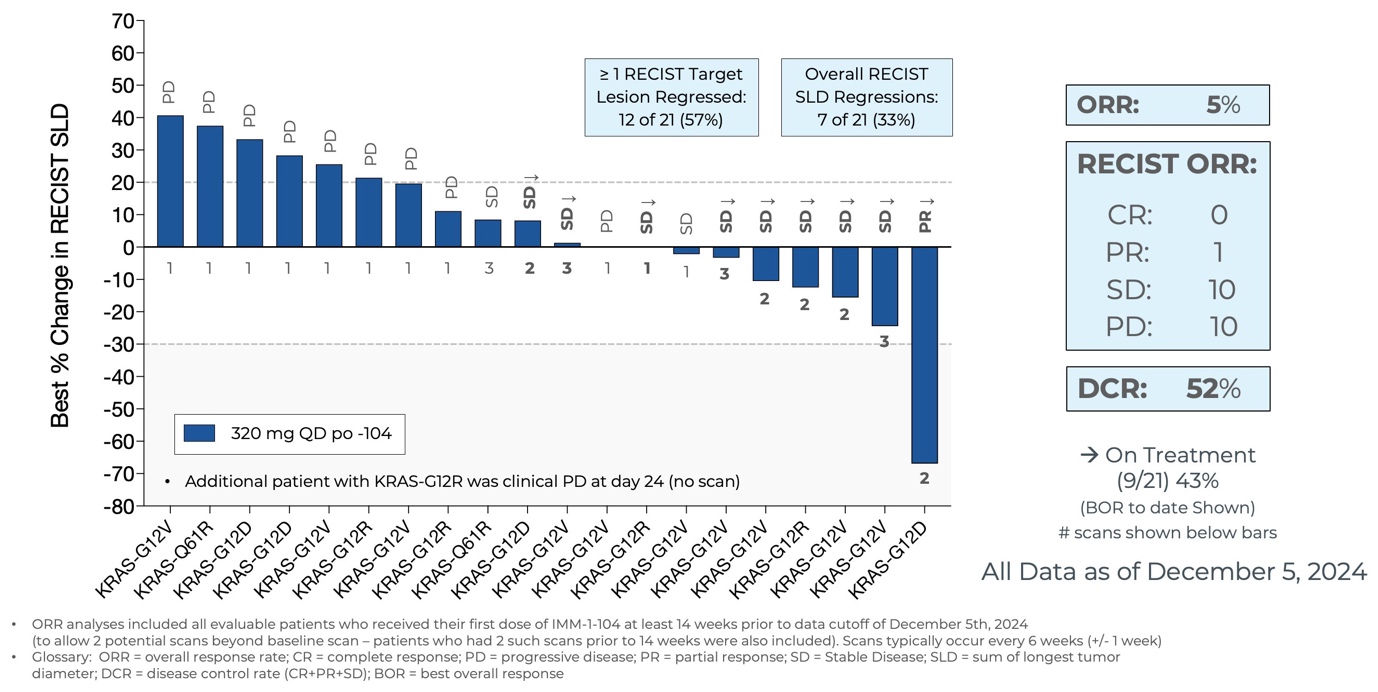
Initial Data from Phase 2a Arm Evaluating IMM-1-104 Monotherapy in Second Line Pancreatic Cancer as of December 5, 2024
Source: Immuneering Corporation
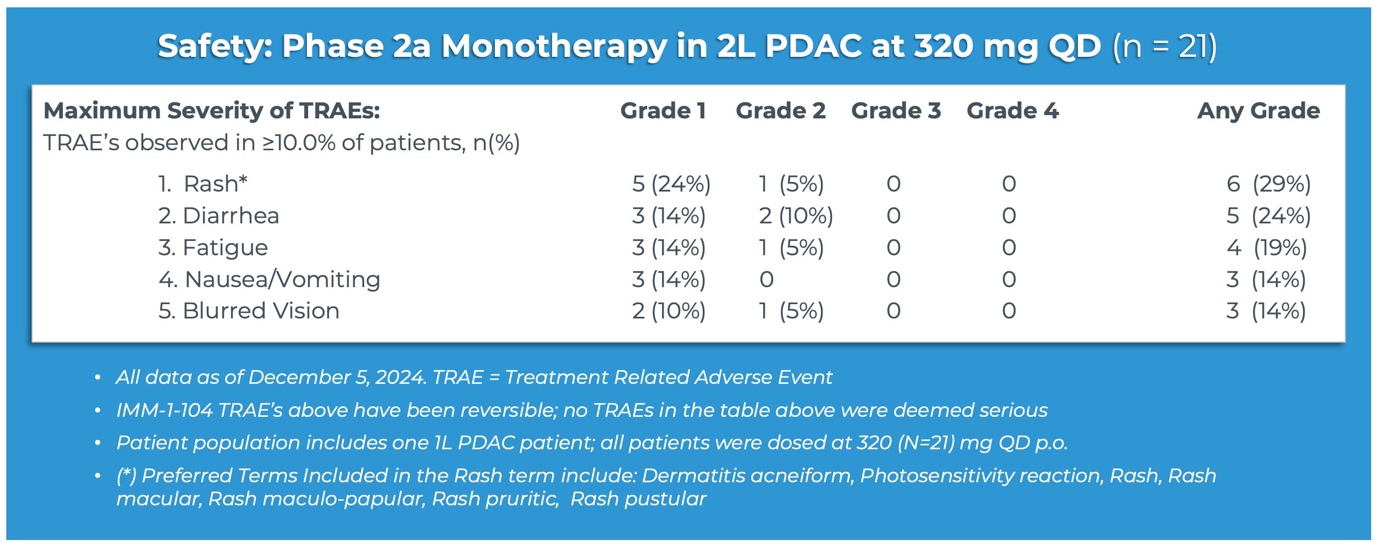
“Having demonstrated compelling activity in both the combination and monotherapy settings for pancreatic cancer, the emerging tolerability profile for IMM-1-104 is also highly promising,” said Brett Hall, Ph.D., Chief Scientific Officer, Immuneering Corporation. “Looking at the table of treatment-related adverse events observed in greater than
Source: Immuneering Corporation
- As of December 5, 2024, eleven of the twenty-one evaluable patients treated in the Phase 2a arm assessing IMM-1-104 as monotherapy in second-line pancreatic cancer achieved disease control, including one patient with
67% target lesion shrinkage (PR). Nine patients remain on treatment. - IMM-1-104 monotherapy was observed to be very well tolerated in second-line pancreatic cancer patients, suggesting that IMM-1-104 may be highly suitable for both monotherapy and combination therapy.
Immuneering previously announced that IMM-1-104 received Fast Track designation from the FDA for the treatment of first- and second-line pancreatic cancer, along with orphan drug designation. The FDA also recently granted Fast Track designation for IMM-1-104 as a treatment for patients with unresectable or metastatic NRAS-mutant melanoma who have progressed on or are intolerant to PD-1/PD-L1 based immune checkpoint inhibitors. Today’s data update follows initial data that was presented in September 2024 on the trial’s arm studying IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer.
Today, Immuneering also announced initial pharmacokinetic, pharmacodynamic and safety data from the Phase 1 portion of the company’s Phase 1/2a trial of IMM-6-415. To date, IMM-6-415 has demonstrated its potential to induce Deep Cyclic Inhibition, and in doing so has been well tolerated – consistent with what was observed preclinically for the development candidate.
Near-Term Milestone Expectations
IMM-1-104
- Further IMM-1-104 Phase 2a data expected in the second quarter of 2025
- Initiation of Phase 2a arm of IMM-1-104 in combination with BRAF inhibitor in melanoma planned for 2025
- Initiation of Phase 2a arms of IMM-1-104 in combination with checkpoint inhibitors in both melanoma and NSCLC planned for 2025
Conference Call
Immuneering will host a conference call and live webcast at 8:30 a.m. ET / 5:30 a.m. PT on January 7, 2025, to discuss the data and provide a business update. Individuals interested in listening to the live conference call may do so by dialing (800) 715-9871 for U.S callers and (646) 307-1963 for other locations and reference conference ID 4497245, or from the webcast link in the “investors” section of the company's website at www.immuneering.com A webcast replay will be available in the investor relations section on the company’s website for 90 days following the completion of the call.
About Immuneering Corporation
Immuneering is a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients. The Company’s lead product candidate, IMM-1-104, is an oral, once-daily deep cyclic inhibitor of MEK designed to improve tolerability and expand indications to include RAS-driven tumors such as most pancreatic cancers. IMM-1-104 is currently in a Phase 1/2a trial in patients with advanced solid tumors including pancreatic cancer. IMM-6-415 is an oral, twice-daily deep cyclic inhibitor of MEK currently in a Phase 1/2a trial in patients with advanced solid tumors harboring RAS or RAF mutations. The company’s development pipeline also includes several early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: Immuneering’s plans to develop, manufacture and commercialize its product candidates; the treatment potential of IMM-1-104 and IMM-6-415, alone or in combination with other agents, including chemotherapy, PD-1 inhibitors and BRAF inhibitors; the future sales of approved MEK inhibitors; the plans and objectives of Company management for future operations, including with respect to the planning and execution of additional IMM-1-104 combination trials and potential pivotal trial of IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel; and the timing for release of additional results from the Phase 2a portion of the trial for IMM-1-104.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding and ability to continue as a going concern; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the period ended September 30, 2024, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Gina Nugent
gina@nugentcommunications.com
Investor Contact:
Laurence Watts
619-916-7620
laurence@newstreetir.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/d2901d24-0359-41be-bfdd-d679a81f408a
https://www.globenewswire.com/NewsRoom/AttachmentNg/229da72c-b1b9-44dc-9da9-b6369e6aca91
https://www.globenewswire.com/NewsRoom/AttachmentNg/59285cbc-ba80-4765-9855-3701ffc9b719
https://www.globenewswire.com/NewsRoom/AttachmentNg/57c609d6-d102-46cf-8a15-706c93aeb8e6








