Enlivex Announces Positive Interim Data Readout from a Phase I/II Trial Evaluating Allocetra in End-Stage Knee Osteoarthritis
Enlivex Therapeutics released positive interim data from a Phase I/II trial of AllocetraTM for end-stage knee osteoarthritis. The trial involved nine patients who received a single injection of AllocetraTM. At three months, the results showed a 64% average reduction in pain, with 33% of patients experiencing complete pain relief. Notably, 89% of patients avoided knee replacement surgery. No severe adverse events were reported. The trial continues to recruit with a goal of 18 patients, assessing safety and pain relief up to 12 months post-injection.
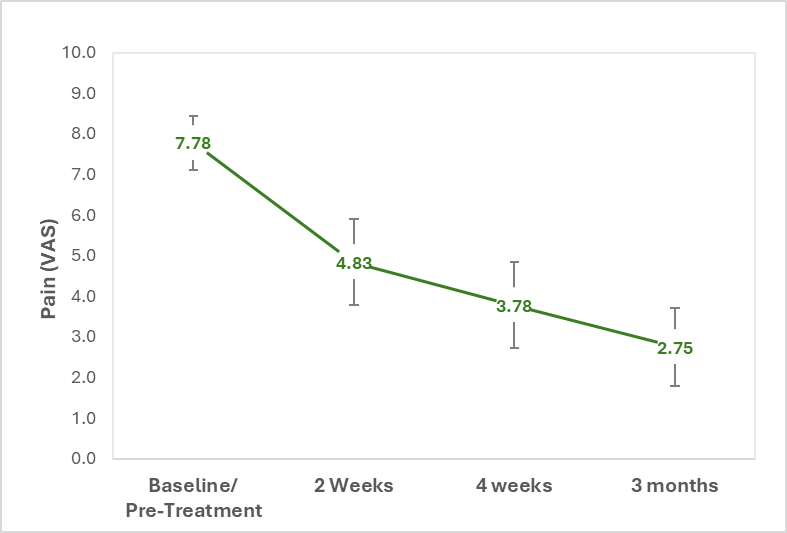
- 64% average reduction in pain reported at three months.
- 33% of patients achieved complete pain relief.
- 89% of patients avoided knee replacement surgery.
- No severe adverse events related to AllocetraTM reported.
- Trial aims to explore further therapeutic uses of AllocetraTM in joint diseases.
- Small sample size of only nine patients so far.
- Only interim data; long-term efficacy and safety still need confirmation.
- One patient (11%) proceeded with knee replacement surgery.
Insights
From a clinical perspective, the interim results for Allocetra™ in treating end-stage knee osteoarthritis are highly promising. A 64% reduction in average pain and 33% reporting complete pain relief are impressive metrics, especially considering these patients had no other viable options. Additionally, the favorable safety profile—with no severe adverse events—suggests Allocetra™ could become a significant alternative to knee replacement surgeries, which are costly and involve lengthy recovery periods.
Evaluating these results, it’s essential to note that sample size is currently small (9 patients). Should these results hold as the study progresses to its full 18-patient enrollment, the broader medical community will likely pay close attention. Future trials with larger samples will be important to confirm the efficacy and safety of Allocetra™. Long-term follow-up will provide insights into the durability of pain relief and functional improvements.
For investors, the data indicates potential for Allocetra™ to significantly impact the osteoarthritis treatment market, potentially reducing the need for invasive surgeries. The outcome of this and subsequent trials will be a litmus test for Enlivex’s future stock performance, as the market seeks tangible results from their innovative approach.
From a financial standpoint, these interim results could trigger a positive shift in Enlivex's stock. The 89% avoidance of knee-replacement surgeries underscores how Allocetra™ could disrupt the market for osteoarthritis treatments, providing a less invasive, more cost-effective alternative. Surgical procedures are not only expensive but also bear significant recovery times, so a viable alternative is likely to attract both patients and healthcare providers.
The short-term implications are clear: positive data readouts often boost investor confidence, leading to potential stock price appreciation. However, for sustained long-term growth, Enlivex will need to move beyond these interim results. The completion of the Phase I/II trial, followed by larger Phase III trials, will be critical. Investors should also watch for any strategic partnerships or licensing deals that could accelerate commercialization and provide additional revenue streams.
Furthermore, if Allocetra™ succeeds in broader applications beyond knee osteoarthritis, such as other joint diseases with inflammatory involvement, the financial upside could be substantial. Yet, one must remain cautiously optimistic until more comprehensive data is available.
Key Highlights
- Enrolled patients with severe knee osteoarthritis who were indicated for knee-replacement surgery received a single local injection of AllocetraTM into the knee as a “last resort”
- Three-month data readout showed a significant reduction in pain and a favorable safety profile
- Pain reduction: patients reported an average pain reduction of
64% from baseline - Complete Pain Resolution:
33% of patients reported complete pain relief, from an average pain level of 9 to a pain level of 0; pain scale used in the study ranged from 0 (no pain) to 10 (maximum pain) - Avoidance of Surgery:
89% of patients did not proceed with knee replacement surgery at three months post-injection - Safety: No severe adverse events related to Allocetra™ were reported
- Pain reduction: patients reported an average pain reduction of
Ness-Ziona, Israel, June 17, 2024 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the "Company"), a clinical-stage macrophage reprogramming immunotherapy company, today released a positive interim data readout from a Phase I/II investigator-initiated clinical trial of AllocetraTM in patients with end-stage knee osteoarthritis who had been indicated for knee replacement surgery.
In this study, patients with end-stage knee osteoarthritis are offered a single AllocetraTM injection to the knee as a potential “last resort” alternative for pain resolution and knee functionality in lieu of knee-replacement surgery. A total of nine patients have been enrolled and treated with a single AllocetraTM injection to the knee and evaluated for at least three months following treatment. Patients reported pain using a scale of zero (0, representing no pain) to ten (10, representing maximum pain).
At the three-month follow up, a substantial reduction (
During the three-month period post injection of AllocetraTM, only a single patient (1/9,
In all cases, dosing was successfully completed, and no severe related adverse events were reported following treatment.
The investigator-initiated trial is led by Amir Oron, M.D., a senior specialist in Orthopedics and Chief of Hand Surgery and Microsurgery at the Kaplan Medical Center in Rehovot, Israel. Dr. Oron stated, "I am pleased with the interim results of this innovative trial, demonstrating the safety of an AllocetraTM injection to the knee in nine patients with severe end-stage knee osteoarthritis. The initial signal of response to the AllocetraTM injection is encouraging, especially as these patients have attempted multiple other treatments with no lasting improvement. These patients have severe osteoarthritis of the knee and need better treatment options to replace or delay progression to extensive surgery.”
Einat Galamidi, M.D., Vice President, Medical of Enlivex stated, "After a robust experience with systemic infusions of AllocetraTM across multiple clinical studies, this is the first report of clinical outcomes following a focused AllocetraTM injection to the joint. We believe that the results confirm the safety of AllocetraTM in the local setting and pave the way to further explore the potential therapeutic utilization of AllocetraTM in joint diseases with inflammatory involvement.”
Recruitment to the study and long-term follow up are ongoing. The study aims to enroll a total of 18 patients to be treated with a single injection of AllocetraTM to the afflicted knee. Patients are assessed for safety following dosing, and pain and function responses to treatment up to 12 months following injection.
Figure 1: Reduction in average pain, reported by patients
ABOUT ALLOCETRA™
Allocetra™ is being developed as a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Diseases such as solid cancers, sepsis, and many others reprogram macrophages out of their homeostatic state. These non-homeostatic macrophages contribute significantly to the severity of the respective diseases. By restoring macrophage homeostasis, Allocetra™ has the potential to provide a novel immunotherapeutic mechanism of action for life-threatening clinical indications that are defined as “unmet medical needs,” as a stand-alone therapy or in combination with leading therapeutic agents.
ABOUT KNEE OSTEOARTHRITIS
Osteoarthritis is by far the most common form of arthritis, affecting more than 32.5 million Americans and more than 300 million individuals worldwide. About half of knees with ACL injuries develop osteoarthritis within 5 to 15 years. 78 million Americans are projected to have osteoarthritis by the year 2040. Symptomatic knee osteoarthritis is particularly prevalent and disabling, with
ABOUT ENLIVEX
Enlivex is a clinical stage macrophage reprogramming immunotherapy company developing Allocetra™, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing and resolution of life-threatening conditions. For more information, visit http://www.enlivex.com.
Safe Harbor Statement: This press release contains forward-looking statements, which may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “would”, “could,” “intends,” “estimates,” “suggests,” “has the potential to” and other words of similar meaning, including statements regarding expected cash balances, market opportunities for the results of current clinical studies and preclinical experiments, the effectiveness of, and market opportunities for, ALLOCETRATM programs. All such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that forward-looking statements involve risks and uncertainties that may affect Enlivex’s business and prospects, including the risks that Enlivex may not succeed in generating any revenues or developing any commercial products; that the products in development may fail, may not achieve the expected results or effectiveness and/or may not generate data that would support the approval or marketing of these products for the indications being studied or for other indications; that ongoing studies may not continue to show substantial or any activity; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward-looking statements. The results of clinical trials in humans may produce results that differ significantly from the results of clinical and other trials in animals. The results of early-stage trials may differ significantly from the results of more developed, later-stage trials. The development of any products using the ALLOCETRATM product line could also be affected by a number of other factors, including unexpected safety, efficacy or manufacturing issues, additional time requirements for data analyses and decision making, the impact of pharmaceutical industry regulation, the impact of competitive products and pricing and the impact of patents and other proprietary rights held by competitors and other third parties. In addition to the risk factors described above, investors should consider the economic, competitive, governmental, technological and other factors discussed in Enlivex’s filings with the Securities and Exchange Commission, including in the Company’s most recent Annual Report on Form 20-F filed with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made, and we do not undertake any obligation to update forward-looking statements, except as required under applicable law.
ENLIVEX CONTACT
Shachar Shlosberger, CFO
Enlivex Therapeutics, Ltd.
shachar@enlivexpharm.com








