Elanco Announces FDA Approval and Launch of Zenrelia™ (ilunocitinib tablets), Offering An Effective, Safe Solution in Canine Dermatology
Elanco Animal Health (NYSE: ELAN) announced FDA approval and launch of Zenrelia™ (ilunocitinib tablets), a once-daily oral JAK inhibitor for dogs with allergic and atopic dermatitis. Zenrelia enters the estimated $1.7 billion global canine dermatology market, offering an effective solution for controlling pruritus in dogs at least 12 months old.
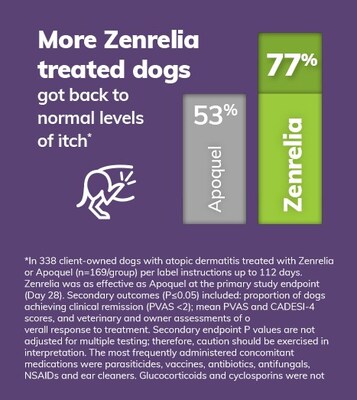
In a head-to-head study, Zenrelia was shown to be at least as effective as the market incumbent JAK inhibitor, with 77% of Zenrelia-treated dogs achieving clinical remission of itch, compared to 53% treated with Apoquel®. Zenrelia's launch price is about 20% less for nearly all dogs compared to the current JAK inhibitor, making it more affordable for pet owners.
The approval represents Elanco's first entry into the fast-growing canine dermatology market, expanding their comprehensive portfolio for veterinarians.
Elanco Animal Health (NYSE: ELAN) ha annunciato l'approvazione da parte della FDA e il lancio di Zenrelia™ (compresse di ilunocitinib), un inibitore JAK orale da somministrare una volta al giorno per cani con dermatite allergica e atopica. Zenrelia entra nel mercato globale della dermatologia canina, stimato in 1,7 miliardi di dollari, offrendo una soluzione efficace per il controllo del prurito nei cani di almeno 12 mesi.
In uno studio diretto, è stato dimostrato che Zenrelia è almeno altrettanto efficace quanto l'inibitore JAK attualmente sul mercato, con il 77% dei cani trattati con Zenrelia che ha raggiunto la remissione clinica del prurito, rispetto al 53% di quelli trattati con Apoquel®. Il prezzo di lancio di Zenrelia è circa il 20% inferiore per quasi tutti i cani rispetto all'attuale inibitore JAK, rendendolo più accessibile per i proprietari di animali domestici.
L'approvazione rappresenta il primo ingresso di Elanco nel mercato della dermatologia canina in rapida crescita, ampliando il loro portfolio completo per i veterinari.
Elanco Animal Health (NYSE: ELAN) anunció la aprobación de la FDA y el lanzamiento de Zenrelia™ (tabletas de ilunocitinib), un inhibidor JAK oral que se administra una vez al día para perros con dermatitis alérgica y atópica. Zenrelia ingresa al mercado global de dermatología canina, estimado en 1.7 mil millones de dólares, ofreciendo una solución efectiva para controlar el prurito en perros de al menos 12 meses.
En un estudio comparativo, se demostró que Zenrelia es al menos tan efectivo como el inhibidor JAK actual en el mercado, con un 77% de los perros tratados con Zenrelia logrando remisión clínica del picor, en comparación con un 53% de los tratados con Apoquel®. El precio de lanzamiento de Zenrelia es aproximadamente un 20% menor para casi todos los perros en comparación con el actual inhibidor JAK, lo que lo hace más asequible para los dueños de mascotas.
La aprobación representa la primera incursión de Elanco en el mercado de dermatología canina de rápido crecimiento, ampliando su cartera integral para veterinarios.
엘란코 애니멀 헬스 (NYSE: ELAN)는 FDA 승인을 발표하고 젠렐리아™ (일루노시티닙 정제)를 출시했습니다. 이 약물은 알레르기성 및 아토피 피부염을 앓고 있는 개를 위한 하루 한 번 복용하는 경구 JAK 억제제입니다. 젠렐리아는 세계 개 피부과 시장(약 17억 달러)에 진입하여 최소 12개월 이상의 개에서 가려움증을 조절하는 효과적인 솔루션을 제공합니다.
직접 비교 연구에서 젠렐리아는 시장에 있는 기존 JAK 억제제만큼 효과적임이 입증되었으며, 젠렐리아로 치료받은 개의 77%가 가려움증의 임상적 완화를 이룬 반면, 아포퀼(Apoquel®)로 치료받은 개는 53%에 불과했습니다. 젠렐리아의 출시 가격은 현재 JAK 억제제에 비해 거의 모든 개에서 약 20% 낮아 애완 동물 소유자에게 더 저렴하게 제공됩니다.
이번 승인은 엘란코가 빠르게 성장하는 개 피부과 시장에 처음 진출하는 것을 의미하며, 수의사를 위한 포괄적인 포트폴리오를 확장합니다.
Elanco Animal Health (NYSE: ELAN) a annoncé l'approbation de la FDA et le lancement de Zenrelia™ (tablettes d'ilunocitinib), un inhibiteur JAK oral à prendre une fois par jour pour les chiens souffrant de dermatite allergique et atopique. Zenrelia entre sur le marché mondial de la dermatologie canine, estimé à 1,7 milliard de dollars, offrant une solution efficace pour contrôler le prurit chez les chiens d'au moins 12 mois.
Dans une étude comparative, Zenrelia s'est révélé au moins aussi efficace que l'inhibiteur JAK déjà présent sur le marché, 77 % des chiens traités avec Zenrelia atteignant une rémission clinique du prurit, contre 53 % traités avec Apoquel®. Le prix de lancement de Zenrelia est environ 20 % inférieur pour presque tous les chiens par rapport à l'inhibiteur JAK actuel, ce qui le rend plus abordable pour les propriétaires d'animaux.
L'approbation représente la première incursion d'Elanco sur le marché en plein essor de la dermatologie canine, élargissant leur portefeuille complet pour les vétérinaires.
Elanco Animal Health (NYSE: ELAN) hat die FDA-Zulassung und den Start von Zenrelia™ (Ilunocitinib-Tabletten) bekannt gegeben, einem einmal täglich einzunehmenden oralen JAK-Inhibitor für Hunde mit allergischer und atopischer Dermatitis. Zenrelia betritt den geschätzten globalen Markt für Hundederma-tologie im Wert von 1,7 Milliarden Dollar und bietet eine effektive Lösung zur Kontrolle von Juckreiz bei Hunden, die mindestens 12 Monate alt sind.
In einer direkten Vergleichsstudie zeigte sich, dass Zenrelia mindestens genauso effektiv ist wie der aktuelle Marktführer unter den JAK-Inhibitoren, wobei 77 % der mit Zenrelia behandelten Hunde eine klinische Remission des Juckreizes erreichten, verglichen mit 53 % der mit Apoquel® behandelten Hunde. Der Einführungspreis von Zenrelia liegt bei nahezu 20 % unter dem des derzeitigen JAK-Inhibitors, was es für Tierhalter erschwinglicher macht.
Die Genehmigung stellt Elancos ersten Eintritt in den schnell wachsenden Markt für Hundederma-tologie dar und erweitert das umfassende Portfolio für Tierärzte.
- FDA approval of Zenrelia for canine allergic and atopic dermatitis
- Entry into estimated $1.7 billion global canine dermatology market
- Zenrelia shown to be at least as effective as market incumbent in head-to-head study
- 77% of Zenrelia-treated dogs achieved clinical remission of itch vs 53% with Apoquel
- Launch price about 20% less than current JAK inhibitor for most dogs
- Expansion of Elanco's comprehensive portfolio for veterinarians
- Boxed warning on safety related to concurrent vaccine administration
- Two dogs were immunosuppressed and euthanized during vaccine response study
Insights
The FDA approval of Zenrelia represents a significant milestone for Elanco, marking their entry into the
The head-to-head study results against Apoquel are particularly promising, with Zenrelia showing superior efficacy in achieving clinical remission of itch (
With a launch price
Elanco's entry into the canine dermatology market with Zenrelia presents a substantial growth opportunity. The global market size of
Investors should note the potential for margin expansion, as specialty pharmaceuticals typically command higher profit margins compared to traditional animal health products. However, the initial marketing and education expenses may impact short-term profitability.
The expansion into dermatology diversifies Elanco's portfolio, potentially reducing revenue volatility. Long-term success will depend on Zenrelia's market penetration and the company's ability to leverage its existing sales channels effectively. Monitor upcoming quarters for early adoption rates and revenue contribution from this new product line.
Zenrelia's approval introduces a valuable new option in canine dermatology. The
However, the boxed warning regarding vaccine administration is a notable concern. This may necessitate careful timing of vaccinations and Zenrelia treatment, potentially complicating management for some patients. Further studies on vaccine response in Zenrelia-treated dogs will be important to refine clinical guidelines.
The reduced risk of "rebound itch" compared to Apoquel is a significant advantage, addressing a common frustration in current treatments. Overall, Zenrelia appears to be a promising addition to our therapeutic arsenal, but its long-term safety profile and real-world efficacy will need ongoing evaluation.
U.S. Food and Drug Administration approves Zenrelia, a once-daily oral JAK inhibitor for dogs with allergic and atopic dermatitis- Elanco enters the estimated
$1.7 billion - In a head-to-head study, Zenrelia was shown to be at least as effective as the market incumbent JAK inhibitor at the primary end point, with an additional endpoint at which Zenrelia got
77% of dogs to clinical remission of itch, compared to53% of dogs treated with Apoquel® (oclacitinib tablet)1* - Zenrelia launch begins in
U.S. with Elanco now taking orders; product expected to ship in coming days
Itching is one of the top reasons pet owners bring their dog to the veterinarian, and pet owners and veterinarians want more canine dermatology options. Approximately 17 million dogs suffer from allergic skin disease, including atopic dermatitis, food allergies or flea sensitivity2. Among pet owners who say their dog's itch is not under control,
The approval of Zenrelia represents an important advancement in treating itchy dogs suffering from chronic, acute or seasonal itch and inflammation in a single, once daily tablet from the start. Zenrelia targets itch where it starts by blocking the pathways involved in allergic itch to break the itch-scratch cycle.4 Zenrelia offers visible improvement from the first dose and minimizes the risk of "rebound itch" which affects many dogs treated with the competitive JAK inhibitor.5-10
"Today is a historic day for Elanco with our first of several expected entries into the fast-growing global canine dermatology market, bringing veterinarians and pet owners a highly effective new solution that got more dogs back to normal levels of itch in a head-to-head study with the current JAK inhibitor on the market1*," said Jeff Simmons, President and CEO, Elanco Animal Health. "We are excited to offer veterinarians and pet owners a solution that can relieve the burdens of itch, while also becoming just the second animal health company to offer veterinarians a comprehensive portfolio, including parasiticides, vaccines, pain and other therapeutics, and now, dermatology."
Promising Results from a Head-to-Head Study1
Elanco conducted a head-to-head noninferiority study comparing the efficacy and safety of Zenrelia and Apoquel for submission in the European Union. The randomized, double-blind study of 338 client-owned dogs with confirmed atopic dermatitis was conducted across 25 study sites in four countries. The study shows one daily dose of Zenrelia is at least as effective as the market incumbent JAK inhibitor at the primary end point on Day 28. Additionally, there were several promising additional endpoints*:
- Zenrelia provided consistently greater relief from itch and skin lesions over time, with once-daily dosing from the start while rebound itch was observed in Apoquel treated dogs after dosing decreased to once daily after Day 14.
- Zenrelia got more dogs back to normal levels of itch by the end of the study, with
77% of Zenrelia treated dogs achieving clinical remission of itch, compared to53% of Apoquel treated dogs. - The difference in treatment response was recognized by owners and veterinarians, with both groups rating Zenrelia higher than Apoquel for overall response to treatment from Day 28 through to Day 112.
- The adverse event profile was similar between the Zenrelia and Apoquel groups.
Veterinarians And Pet Owners Need More Options
Research shows that nearly
"We're dedicated to solving these unmet needs in canine dermatology," said Bobby Modi, Executive Vice President,
Take for example, Trooper, a one-year-old Yorkshire Terrier who enrolled in the Zenrelia clinical trial. Prior to participating in the clinical trial, Trooper's itch level was 10 out of 10 on the pruritus visual analog scale (PVAS), a validated observation scale for canine itch. By the end of the first two weeks of treatment, he was back to a normal itch level of 1.9 and finished the clinical trial with an itch level of 1.1.** A PVAS score of less than 2 is considered a normal level of itch, also referred to as clinical remission of itch. You can read more about Trooper's story here.
"I was excited to participate as a clinical investigator in the Zenrelia field study because it is clear we need more treatment options for itchy dogs," said Dr. Tom Lewis, veterinarian and founder of Dermatology for Animals, a group of veterinary dermatology clinics committed to caring for pets with allergies. "I saw amazing results during the clinical field study and am eager to get many patients started on Zenrelia. Watching dogs get back to normal quickly and seeing the bond restored between the dog and pet parent was incredibly rewarding."
Demonstrated Safety
The safety of Zenrelia has been demonstrated in multiple toxicity and clinical safety studies. The required margin of safety study for Zenrelia was conducted in healthy dogs dosed with placebo, 1, 2, 3 or 5 times the label dose daily for six months. All dogs completed the study with no serious adverse events.
The Zenrelia label includes a boxed warning on safety related to concurrent vaccine administration based on the results of a vaccine response study. In this study, eight, 10-month-old laboratory beagles received primary vaccinations while being treated with Zenrelia at 3X the label dose. Two dogs were immunosuppressed and euthanized during the study. Antibody responses were evaluated following vaccination. All but one dog responded successfully to modified live vaccines, and two of six dogs responded to inactivated Rabies vaccine at the primary endpoint.
Dogs should be up to date on vaccinations prior to starting Zenrelia. It's important for veterinarians to read the entire package insert, including the Boxed Warning, before prescribing Zenrelia.
"Zenrelia has been demonstrated to be safe and highly effective in a number of studies," said Dr. Mara Tugel, veterinarian and Dermatology Medical Strategic Lead at Elanco. "We recognize that veterinarians need clinically relevant data to guide treatment choices, and plan to pursue additional studies to evaluate vaccine response in Zenrelia-treated dogs. We will continue to work to improve the label over time."
Veterinarians in the
Elanco will conduct a conference call on Friday, September 20, 2024 at 8:00 am eastern time to discuss the Zenrelia approval with the investment community and other interested parties. A live webcast of the conference call can be accessed through the link that will be posted on Elanco's website at https://investor.elanco.com/events-and-presentations/default.aspx. A replay will also be available on the website shortly following the call.
ABOUT ELANCO
Elanco Animal Health Incorporated (NYSE: ELAN) is a global leader in animal health dedicated to innovating and delivering products and services to prevent and treat disease in farm animals and pets, creating value for farmers, pet owners, veterinarians, stakeholders and society as a whole. With nearly 70 years of animal health heritage, we are committed to helping our customers improve the health of animals in their care, while also making a meaningful impact on our local and global communities. At Elanco, we are driven by our vision of Food and Companionship Enriching Life and our Elanco Healthy Purpose™ sustainability pillars – all to advance the health of animals, people, the planet and our enterprise. Learn more at www.elanco.com.
INDICATIONS
is indicated for control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age.
IMPORTANT SAFETY INFORMATION
Read the entire package insert before using this drug, including the boxed warning. For Full prescribing information call 1 888 545 5973 or visit www.elancolabels.com/us/zenrelia.
WARNING: VACCINE-INDUCED DISEASE AND INADEQUATE IMMUNE RESPONSE TO VACCINES. Based on results of the vaccine response study, dogs receiving Zenrelia are at risk of fatal vaccine-induced disease and inadequate immune response to vaccines. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination. Dogs should be up to date on vaccinations prior to starting Zenrelia. Do not use in dogs less than 12 months old or dogs with a serious infection. Monitor dogs for infections because Zenrelia may increase susceptibility to opportunistic infections. Neoplastic conditions (benign and malignant) were observed during clinical studies. Consider the risks and benefits of treatment in dogs with a history of recurrence of these conditions. The most common adverse reactions were vomiting, diarrhea and lethargy. Zenrelia has not been evaluated in breeding, pregnant, or lactating dogs and concurrent use with glucocorticoids, cyclosporine, or other systemic immunosuppressive agents has not been tested. For full prescribing information see package insert. Forward-Looking Statements: TBD
Zenrelia, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates. Apoquel is a trademark of Zoetis Services, LLC. © 2024 Elanco or its affiliates.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws, including, without limitation, statements concerning Zenrelia as a treatment for dogs with allergic dermatitis and timeline for launch, commercial uptake, as well as future studies, publications and other milestones related to Zenrelia, and reflects Elanco's current beliefs and expectations. However, as with any animal health pharmaceutical product, there are substantial risks and uncertainties in the process of drug research, development, and commercialization. Among other things, there is no guarantee that planned or ongoing studies will be completed as planned, that future study results will be consistent with study results to date, that future studies will receive regulatory approval, or that Elanco will execute its strategy as expected. For further discussion of these and other risks and uncertainties that could cause actual results to differ from Elanco's expectations, see Elanco's Form 10-K and Form 10-Q filings with the United States Securities and Exchange Commission. Except as required by law, Elanco undertakes no duty to update forward-looking statements to reflect events after the date of this release.
Investor Contact: Katy Grissom (317) 273-9284 kathryn.grissom@elancoah.com
Media Contact: Season Solorio (765) 316-0233 season.solorio@elancoah.com
References:
- Elanco Animal Health. Data on File.
- AVMA Sourcebook. Elanco, Dermatology Products SOV Study, 2023
- Elanco and FleishmanHillard TRUE Global Intelligence Survey.
- Elanco Animal Health. Data on File.
- Cosgrove SB, et al. A blinded, randomized, placebo-controlled trial of efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet Dermatol, 2013; 24: 587-e142.
- Little PR, et al. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client-owned dogs. Vet Dermatol, 2015; 26: 23-e8.
- Fukuyama T, et al. Demonstration of rebound phenomenon following abrupt withdrawal of the JAK1 inhibitor oclacitinib. European Journal of Pharmacology, 2017; 794: 20-26.
- Takahashi J, et al. Efficacy and safety of
0.0584% hydrocortisone aceponate topical spray and systemic oclacitinib combination therapy in dogs with atopic dermatitis: a randomized, double-blinded, placebo-controlled trial. Vet Dermatol, 2021; 32: 119-e35. - Denti D, et al. Prolonged twice-daily administration of oclacitinib for the control of canine atopic dermatitis: a retrospective study of 53 client-owned atopic dogs. Vet Dermatol, 2022; 33: 149-e42.
- Olivry T, et al. A randomized controlled trial testing the rebound-preventing benefit of four days of prednisolone during the induction of oclacitinib therapy in dogs with atopic dermatitis. Vet Dermatol, 2023; 34: 99-106.
*Zenrelia was as effective as Apoquel at the primary study endpoint (Day 28). Additional outcomes (P≤0.05) included: proportion of dogs achieving PVAS <2; Mean PVAS and CADESI-4 scores, and veterinary and owner assessments of overall response to treatment. Additional endpoint P values are not adjusted for multiple testing; therefore, caution should be exercised in interpretation.
** Individual results may vary. In the
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/elanco-announces-fda-approval-and-launch-of-zenrelia-ilunocitinib-tablets-offering-an-effective-safe-solution-in-canine-dermatology-302253609.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/elanco-announces-fda-approval-and-launch-of-zenrelia-ilunocitinib-tablets-offering-an-effective-safe-solution-in-canine-dermatology-302253609.html
SOURCE Elanco Animal Health