Desktop Health® Announces Flexcera® Smile Ultra+ Dental Resin Validated for All-on-X Implant Provisionals
- Launched in 2021, Flexcera is a family of popular, FDA 510(k) cleared nanoceramic polymers used for 3D printing restorative and removable dental products
- Flexcera Smile Ultra+ dental resin is an essential All-on-X product, enabling dental professionals to deliver strong and lifelike implant-supported denture prosthetics quickly for the aging edentulous population
- The estimated number of edentulous Americans, defined as the complete loss of all teeth, is greater than 36 million
-
The global dental implant and prosthetics market is poised to reach
$16B 7.5% from 2023 to 2029, driven by increasing number of elderly individuals who often require dental restorations and implants - Videos showing dental labs and dentists using Flexcera for All-on-X and other restorative cases can be seen at TeamDM.com/FlexceraSmiles
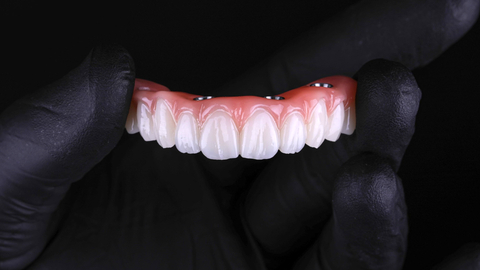
Flexcera Smile Ultra+ dental resin is now validated for implant-supported denture provisionals, also known as “All-on-X” prosthetics. All-on-X image courtesy of Absolute Dental Services in Durham, NC. (Photo: Business Wire)
Flexcera Smile Ultra+ dental resin is an FDA (510)k cleared, MDR certified, and CE marked resin for 3D printed restoratives, such as crowns and bridges, and removables, such as dentures. Now this popular and versatile material is indicated for implant-supported denture provisionals, which is commonly referred to as an “All-on-X” prosthetic. The “X” is a patient-specific variable for how many implants are supporting the denture.
All-on-X provisionals are increasingly a popular same-day dentistry solution for implant specialists and dental laboratories as use of intraoral scanners and computer aided design in dentistry has grown, enabling rapid design and manufacturing. Available in six natural tooth shades and in two bottle sizes, Flexcera Smile Ultra+ has become a go-to product for provisionals as it prints quickly and accurately – giving patients a beautiful, functional smile as they heal for their final restoration.
A full set of Flexcera upper teeth arches costs a fraction of other handmade or milled solutions on the market. A single kilogram bottle of Flexcera Smile Ultra+ delivers up to 50 affordable All-on-X arches. This allows dental professionals to print multiple copies and personalize the patient shades, delivering next-level patient care.
Flexcera Smile Ultra+ is validated for 3D printing on the following dental printers:
- Desktop Health® Einstein, Einstein Pro XL, EnvisionOne, and D4K
- Asiga® Max UV, Max 2, Ultra, and Pro 4K
- Carbon® M series dental 3D printers
Print time for a full set of Flexcera Smile Ultra+ arches is about 20-30 minutes depending on case requirements and printer used.
Flexcera Smiles a Popular All-on-X Solution
Dental labs and dentists say Flexcera provisionals satisfy an important need for patients.
“We print All-on-X provisionals with Flexcera Smile Ultra+ all day to meet the demand of our digital doctors,” said Frankie Acosta, Owner and Dental Technician, at AA Dental Design, in
Dr. Rami Jandali, a board certified Prosthodontist at Amazing Smiles in
Dr. Bruce Smoler of Smoler Smiles in
To learn more, visit TeamDM.com/Flexcera
About Desktop Metal
Desktop Metal (NYSE:DM) is driving Additive Manufacturing 2.0, a new era of on-demand, digital mass production of industrial, medical, and consumer products. Our innovative 3D printers, materials, and software deliver the speed, cost, and part quality required for this transformation. We’re the original inventors and world leaders of the 3D printing methods we believe will empower this shift, binder jetting and digital light processing. Today, our systems print metal, polymer, sand and other ceramics, as well as foam and recycled wood. Manufacturers use our technology worldwide to save time and money, reduce waste, increase flexibility, and produce designs that solve the world’s toughest problems and enable once-impossible innovations. Learn more about Desktop Metal and our #TeamDM brands at www.desktopmetal.com.
Forward-looking Statements
This press release contains certain forward-looking statements within the meaning of the federal securities laws, including statements about Desktop Metal’s strategic integration and cost savings initiatives, expected restructuring charges, anticipated cost savings, long-term growth, market share, liquidity and profitability, are forward-looking statements. Forward-looking statements generally are identified by the words “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this document, including but not limited to, the risks and uncertainties set forth in Desktop Metal, Inc.'s filings with the
View source version on businesswire.com: https://www.businesswire.com/news/home/20240729235450/en/
Investor Relations:
(857) 504-1084
DesktopMetalIR@icrinc.com
Media Relations:
Sarah
(724)516-2336
Sarahwebster@desktopmetal.com
Source: Desktop Health







