ZEISS innovation paves way to more personalized patient care with cutting-edge AI technology and industry-leading surgical solutions
ZEISS Medical Technology is showcasing its latest innovations at the ESCRS conference, highlighting advancements in cataract and corneal refractive workflows. Key developments include:
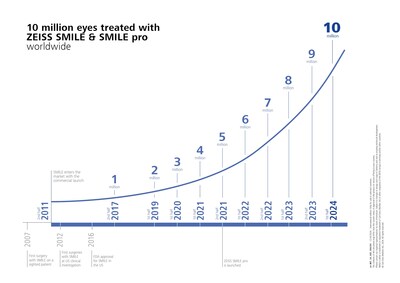
1. ZEISS SMILE pro for hyperopia treatment in CE markets, with over 10 million eyes treated using ZEISS SMILE technology.
2. Introduction of advanced optical and digital ophthalmic microscopes: ZEISS ARTEVO 750 and ZEISS ARTEVO 850.
3. New clinical results for the AT ELANA 841P trifocal IOL.
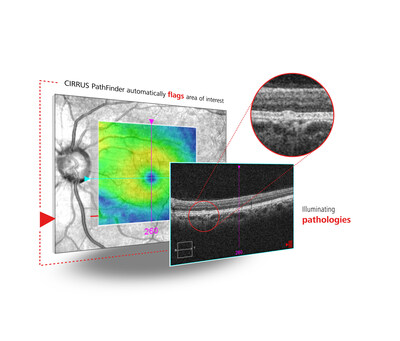
4. AI advancements: ZEISS AI IOL Calculator (CE-marked) and CIRRUS PathFinder (pending CE mark) for improved pre-operative patient care.
5. ZEISS Surgery Optimizer with AI-based video segmentation for post-surgery analysis.
These innovations aim to enhance surgical workflows, improve patient outcomes, and pave the way for more personalized care in ophthalmology.
ZEISS Medical Technology è in mostra con le sue ultime innovazioni alla conferenza ESCRS, evidenziando i progressi nei flussi di lavoro per la cataratta e la chirurgia refrattiva corneale. I principali sviluppi includono:
1. ZEISS SMILE pro per il trattamento della ipermetropia nei mercati CE, con oltre 10 milioni di occhi trattati utilizzando la tecnologia ZEISS SMILE.
2. Introduzione di microscopi oftalmici ottici e digitali avanzati: ZEISS ARTEVO 750 e ZEISS ARTEVO 850.
3. Nuovi risultati clinici per l'AT ELANA 841P trifocal IOL.
4. Progressi nell'IA: ZEISS AI IOL Calculator (marchiato CE) e CIRRUS PathFinder (in attesa di marchio CE) per migliorare la cura dei pazienti pre-operatori.
5. ZEISS Surgery Optimizer con segmentazione video basata sull'IA per l'analisi post-chirurgica.
Queste innovazioni mirano a migliorare i flussi di lavoro chirurgici, migliorare i risultati per i pazienti e aprire la strada a cure più personalizzate in oftalmologia.
ZEISS Medical Technology está mostrando sus últimas innovaciones en la conferencia ESCRS, destacando mejoras en los flujos de trabajo de cataratas y cirugía refractiva corneal. Los desarrollos clave incluyen:
1. ZEISS SMILE pro para el tratamiento de la hipermetropía en mercados CE, con más de 10 millones de ojos tratados utilizando la tecnología ZEISS SMILE.
2. Introducción de microscopios oftálmicos ópticos y digitales avanzados: ZEISS ARTEVO 750 y ZEISS ARTEVO 850.
3. Nuevos resultados clínicos para el AT ELANA 841P trifocal IOL.
4. Avances en IA: ZEISS AI IOL Calculator (marcado CE) y CIRRUS PathFinder (pendiente de marcado CE) para mejorar la atención del paciente preoperatorio.
5. ZEISS Surgery Optimizer con segmentación de video basada en IA para el análisis postoperatorio.
Estas innovaciones tienen como objetivo mejorar los flujos de trabajo quirúrgicos, mejorar los resultados de los pacientes y allanar el camino para una atención más personalizada en oftalmología.
ZEISS Medical Technology는 ESCRS 회의에서 최신 혁신 기술을 선보이며 백내장 및 각막 굴절 수술의 작업 흐름에서의 발전을 강조하고 있습니다. 주요 개발 사항은 다음과 같습니다:
1. CE 시장에서의 원시 교정을 위한 ZEISS SMILE pro, ZEISS SMILE 기술을 통해 1천만 개 이상의 눈이 치료되었습니다.
2. 첨단 광학 및 디지털 안과 현미경의 도입: ZEISS ARTEVO 750와 ZEISS ARTEVO 850.
3. AT ELANA 841P 삼중 초점 인공 수정체에 대한 새로운 임상 결과.
4. AI의 발전: 수술 전 환자 관리를 개선하기 위한 ZEISS AI IOL Calculator (CE 마크 획득) 및 CIRRUS PathFinder (CE 마크 대기 중).
5. 수술 후 분석을 위한 AI 기반 비디오 분할 기능을 갖춘 ZEISS Surgery Optimizer.
이러한 혁신은 수술 작업 흐름을 개선하고 환자 결과를 향상시키며 안과에서 보다 개인화된 치료의 길을 열기 위해 설계되었습니다.
ZEISS Medical Technology présente ses dernières innovations lors de la conférence ESCRS, mettant en avant les avancées dans les flux de travail liés à la cataracte et à la chirurgie réfractive cornéenne. Les développements clés incluent :
1. ZEISS SMILE pro pour le traitement de l'hypermétropie sur les marchés CE, avec plus de 10 millions d'yeux traités grâce à la technologie ZEISS SMILE.
2. Introduction de microscopes ophtalmiques optiques et numériques avancés : ZEISS ARTEVO 750 et ZEISS ARTEVO 850.
3. Nouveaux résultats cliniques pour le AT ELANA 841P trifocal IOL.
4. Progrès en IA : ZEISS AI IOL Calculator (marqué CE) et CIRRUS PathFinder (en attente de marquage CE) pour une meilleure prise en charge des patients avant l'opération.
5. ZEISS Surgery Optimizer avec segmentation vidéo basée sur l'IA pour l'analyse post-opératoire.
Ces innovations visent à améliorer les flux de travail chirurgicaux, à optimiser les résultats pour les patients et à ouvrir la voie à des soins plus personnalisés en ophtalmologie.
ZEISS Medical Technology präsentiert auf der ESCRS-Konferenz seine neuesten Innovationen und hebt Fortschritte in den Arbeitsabläufen bei Katarakt- und Hornhaut-Refraktiveingriffen hervor. Zu den wichtigsten Entwicklungen gehören:
1. ZEISS SMILE pro zur Behandlung von Hyperopie in CE-Märkten, mit über 10 Millionen behandelten Augen mit ZEISS SMILE-Technologie.
2. Einführung fortschrittlicher optischer und digitaler ophthalmologischer Mikroskope: ZEISS ARTEVO 750 und ZEISS ARTEVO 850.
3. Neue klinische Ergebnisse für die AT ELANA 841P trifokal IOL.
4. KI-Entwicklungen: ZEISS AI IOL Calculator (CE-zertifiziert) und CIRRUS PathFinder (CE-Zertifizierung ausstehend) zur Verbesserung der präoperativen Patientenversorgung.
5. ZEISS Surgery Optimizer mit KI-basierter Video-Segmentierung für die Analyse nach der Operation.
Diese Innovationen zielen darauf ab, die chirurgischen Arbeitsabläufe zu verbessern, die Ergebnisse für die Patienten zu optimieren und den Weg für personalisierte Pflege in der Augenheilkunde zu ebnen.
- ZEISS SMILE pro introduces hyperopia treatment with lenticule extraction in CE markets
- Over 10 million eyes treated with ZEISS SMILE and ZEISS SMILE pro technology
- Launch of advanced optical and digital ophthalmic microscopes ZEISS ARTEVO 750 and 850
- CE mark approval for ZEISS AI IOL Calculator for data-driven IOL power calculations
- Introduction of CIRRUS PathFinder AI tool for pre-operative cataract patient assessment (pending CE mark)
- None.
Showcasing at ESCRS from Sept. 6 to 10, in
REFRACTIVE INDUSTRY MILESTONES: ZEISS extends its global refractive industry momentum with more than 10 million eyes treated with ZEISS SMILE and ZEISS SMILE pro; ZEISS announces first treatment in the CE market for hyperopia with lenticule extraction using ZEISS SMILE pro technology.
NEW DIGITAL & SURGICAL ENHANCEMENTS IN THE OR: Expanded portfolio of advanced optical and digital ophthalmic microscopes introduced in CE markets with ZEISS ARTEVO 750 and ZEISS ARTEVO 850.
EXCELLENT IOL CLINICAL RESULTS: ZEISS showcases its advanced ZEISS IOL portfolio, including new clinical results with the AT ELANA 841P trifocal IOL and its innovative ZEISS trifocal technology, highlighting excellent visual outcomes.
AI ADVANCEMENTS FOR CATARACT PATIENTS: ZEISS brings data-driven IOL power calculations to European cataract market with CE mark for the ZEISS AI IOL Calculator; ZEISS CIRRUS PathFinder provides fully integrated AI decision support through automated OCT assessment for pre-operative cataract patients (pending CE mark); ZEISS Surgery Optimizer features AI-based video segmentation to review, analyze, and share surgery videos post-surgery.
JENA,
"ZEISS continues to pave the way toward a future of truly personalized care through the development of some of the most advanced digital technologies and medical devices to meet the expanding needs of
Leading the next era in laser vision correction
After more than 13 years of offering laser vision correction (LVC) solutions, including treatment options for myopia and presbyopia, ZEISS is once again reshaping the market with the introduction of lenticule extraction for hyperopic patients, with or without astigmatism, using the VISUMAX® 800. ZEISS is the first medical device manufacturer to introduce treatment of hyperopia using lenticule extraction in the CE market.
At ESCRS, ZEISS is also showcasing its complete ZEISS Corneal Refractive Workflow, now further enhanced by including computer-assisted cyclotorsion adjustment for the ZEISS VISUMAX 800, remote planning with the ZEISS Refractive Workplace, and the VISULYZE® clinical nomogram and data analysis tool, all further enhancing the ZEISS digital portfolio and workflow solutions for corneal refractive. A recent study found that lenticule extraction using a ZEISS femtosecond laser provides an effective treatment method for the correction of compound hyperopic astigmatism, demonstrating a high level of efficacy, predictability, safety, and stability.1
"ZEISS SMILE has become a benchmark for refractive surgery because of its quality and the results. In maybe 20 to 30 seconds, you change peoples' lives," says Dr. Andrea Russo, MD, Ph.D. Bio, Centro Oculistico Bresciano in
Demonstrating its continued momentum in the LVC market, ZEISS announced that more than 10 million eyes have now been successfully treated with lenticule extraction solutions utilizing ZEISS SMILE and ZEISS SMILE pro, marking a significant milestone for the company and proof of the growing international adoption of safe and effective lenticule extraction solutions. In 2011, ZEISS was the first medical device manufacturer to make lenticule extraction for laser vision correction commercially available, and ZEISS SMILE and ZEISS SMILE pro continue to be leading solutions trusted by surgeons for the technology's proven reliability and effective treatment with the VisuMax® and VISUMAX® 800 from ZEISS.
At ESCRS, three new research abstracts will be presented that highlight the findings of a recent post-market clinical follow-up study conducted by ZEISS, which examined 237 patients who were treated with ZEISS SMILE pro using the ZEISS VISUMAX 800 femtosecond laser. Key findings will be highlighted in the following three abstracts being presented:
- "Mesopic Contrast Sensitivity Evaluation after Small-Incision Lenticule Extraction using the VISUMAX 800 in a Post-Market Clinical Follow Up Study" – presented by Prof. Jesper Hjortdal
- "Incidence of Suction Loss and Speed of Lenticule Creation with Small-Incision Lenticule Extraction using the VISUMAX 800 in a Post-Market Clinical Follow Up Study" – presented by Dr. Rainer Wiltfang
- "Early Visual Recovery and Energy Settings After Small-Incision Lenticule Extraction Using the VISUMAX 800 In A Post-Market Clinical" – presented by Dr. Walter Sekundo
Digital visualization enhancements elevate the surgical workflow
ZEISS is continuing to roll-out its expanded portfolio of high-end microscopes with the ZEISS ARTEVO® 850 3D heads-up ophthalmic microscope setting the pace in digital visualization with true color imaging and increased depth of field by nearly 60 percent.2 Additionally, the ZEISS ARTEVO 850 with CALLISTO eye® features a redesigned intuitive user interface that centralizes all controls on a single touchscreen. The second latest addition to ZEISS's portfolio of optical surgical microscopes, the new ARTEVO® 750, elevates the surgical workflow by introducing advanced optical visualization technology, including new RGB LED illumination with adjustable light color temperature, as well as a 40 percent increase3 in resolution of data overlays provided in the eyepiece. The two devices are CE and FDA cleared and commercially available in all major markets.
Excellent clinical results with the new AT ELANA 841P trifocal IOL
Within the ZEISS Premium Cataract Workflow, the novel trifocal intraocular lens (IOL), AT ELANA® 841P, represents the best of ZEISS trifocal technology on a glistening-free hydrophobic c-loop platform. At ESCRS, new clinical results with the ZEISS AT ELANA 841P will be presented, highlighting excellent visual outcomes:
- "First Clinical Results of a Multicentric Prospective Study on the Visual Performance of Patients Implanted with the Hydrophobic Trifocal AT ELANA 841P Intraocular Lens" – presented by Dr. Florian Kretz
- "First Clinical Results of a Multicentric Prospective Study on The Visual Performance of Patients Implanted with a Hydrophobic Trifocal IOL" – presented by Dr. Ramin Khoramnia
The power of AI supports surgeons with integrated tools for their cataract workflow
ZEISS continues to offer new digital tools to assist surgeons throughout their cataract workflow. The ZEISS AI IOL Calculator recently received its CE mark and will be available on the digital surgery planner ZEISS EQ Workplace. It is also compatible with the ZEISS IOLMaster 700 Total Keratometry (TK®). The ZEISS AI IOL Calculator is a data driven IOL power calculation algorithm and is a core feature of the ZEISS Cataract Workflow. It is optimized with a large amount of data for each IOL model it supports and therefore does not require IOL constants. It has shown great performance when applied to short eyes and outperformed most state-of-the-art formulas in short eyes.4
"What's so unique about ZEISS AI IOL Calculator is that it is IOL specific but does not require IOL constants. We rely on the ZEISS AI IOL Calculator for the entire spectrum of axial lengths, and it especially shines in short eyes," said Dr. Douglas D. Koch, M.D.,
As an example of the company's digital diagnostic OCT leadership, ZEISS will also show ZEISS CIRRUS PathFinder, a fully integrated AI decision support tool that uses proprietary deep learning algorithms, trained by retina specialists, to automatically identify abnormal macular OCT B-scans assisting with pre-surgical assessment of the retina and optimizing outcomes. ZEISS CIRRUS PathFinder will be available in select markets subject to local regulatory clearances and is currently pending CE mark.5
In addition, ZEISS will demonstrate how post-surgery review, analysis and sharing of surgery videos is now made possible with a new spatial computing app for reviewing 3D videos, based on the ZEISS Surgery Optimizer. This exclusive experience on Apple Vision Pro represents an entirely new way for ophthalmologists to review 3D videos pre-recorded with ZEISS digital surgical microscopes. Using an Apple Vision Pro, surgeons can review 2D or 3D surgery videos, scans of the eye, patient information and much more simultaneously; a comprehensive spatial computing experience easily controlled by the user's eyes, hands, and voice.
ZEISS will showcase its latest innovations across the cataract and corneal refractive workflows at ESCRS in Hall 7 at booth D23 from Sept. 6-10, in
1 Based on data generated within a designated clinical investigation conducted with predecessor ZEISS VisuMax: Reinstein DZ, Sekundo W, Archer TJ, Stodulka P, Ganesh S, Cochener B, Blum M, Wang Y, Zhou X. SMILE for Hyperopia With and Without Astigmatism: Results of a Prospective Multicenter 12-Month Study. J Refract Surg. 2022 Dec;38(12):760-769. doi: 10.3928/1081597X-20221102-02. Epub 2022 Dec 1. PMID: 36476297.
2 Compared to ZEISS ARTEVO 800.
3 Compared to previous generation ZEISS LUMERA 700. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems.
4 Kenny et al. Efficacy of segmented axial length and artificial intelligence approaches to intraocular lens power calculation in short eyes. J Cataract Refract Surg. 2023 Jul.
5 PathFinder is compatible with all current CIRRUS devices from ZEISS: 500, 5000, 6000, pending local regulatory clearances.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development.
Contact for investors
Sebastian Frericks
Director Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: investors.meditec@zeiss.com
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 4,823 employees worldwide, the Group generated revenue of
The Group's head office is located in Jena,
For further information visit: www.zeiss.com/med
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-innovation-paves-way-to-more-personalized-patient-care-with-cutting-edge-ai-technology-and-industry-leading-surgical-solutions-302233624.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-innovation-paves-way-to-more-personalized-patient-care-with-cutting-edge-ai-technology-and-industry-leading-surgical-solutions-302233624.html
SOURCE Carl Zeiss Meditec AG