ZEISS expands ophthalmic offerings to improve patient care with new digital AI tools and revolutionary surgical solutions
ZEISS Medical Technology is showcasing new digital enhancements and surgical solutions at the American Academy of Ophthalmology (AAO) conference from Oct. 19-21, 2024, in Chicago. Key highlights include:
- Introduction of ZEISS VisioGen, an AI-driven solution for enhancing refractive patient communication and streamlining clinic operations
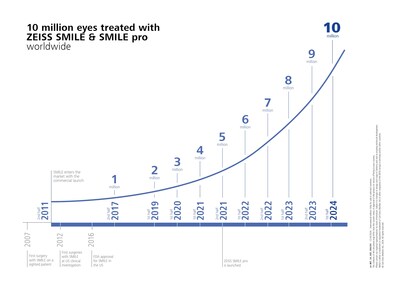
- Celebration of 10 million eyes treated with ZEISS SMILE and ZEISS SMILE pro lenticule extraction solutions
- Marking 25 years of leadership in optical biometry with ZEISS IOLMaster biometers
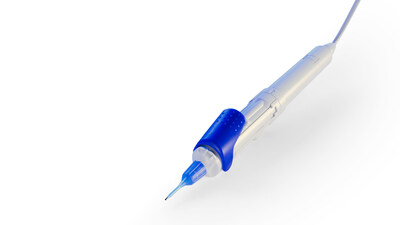
- Demonstration of the FDA-cleared ZEISS MICOR 700, the first hand-held lens removal device with ultrasound-free operation
- Expansion of the ZEISS Retina Workflow to include advanced vitreoretinal surgical solutions from DORC
- FDA clearance for next-generation ZEISS VISULAS combi and green therapeutic lasers
These innovations aim to advance clinical workflows and support personalized care across cataract, corneal refractive, retina, and glaucoma treatments.
ZEISS Medical Technology presenta nuove innovazioni digitali e soluzioni chirurgiche alla conferenza dell'American Academy of Ophthalmology (AAO) che si terrà dal 19 al 21 ottobre 2024 a Chicago. I punti salienti includono:
- Introduzione di ZEISS VisioGen, una soluzione guidata dall'AI per migliorare la comunicazione con i pazienti refrattivi e ottimizzare le operazioni cliniche
- Celebrazione di 10 milioni di occhi trattati con le soluzioni di estrazione lenticolare ZEISS SMILE e ZEISS SMILE pro
- Commemorazione di 25 anni di leadership nella biomettria ottica con i biometria ZEISS IOLMaster
- Dimostrazione del ZEISS MICOR 700 approvato dalla FDA, il primo dispositivo portatile per la rimozione di lenti con operazione senza ultrasuoni
- Espansione del ZEISS Retina Workflow per includere avanzate soluzioni chirurgiche vitreoretinali di DORC
- Approvazione della FDA per i laser terapeutici di nuova generazione ZEISS VISULAS combi e laser verdi
Queste innovazioni mirano a sviluppare i flussi di lavoro clinici e supportare la cura personalizzata in ambito di cataratta, rifrazione corneale, retina e trattamenti per il glaucoma.
ZEISS Medical Technology está presentando nuevas mejoras digitales y soluciones quirúrgicas en la conferencia de la American Academy of Ophthalmology (AAO) que se llevará a cabo del 19 al 21 de octubre de 2024 en Chicago. Los aspectos destacados incluyen:
- Introducción de ZEISS VisioGen, una solución impulsada por IA para mejorar la comunicación con pacientes refractivos y optimizar las operaciones clínicas
- Celebración de 10 millones de ojos tratados con las soluciones de extracción lenticular ZEISS SMILE y ZEISS SMILE pro
- Conmemoración de 25 años de liderazgo en biometía óptica con los biometría ZEISS IOLMaster
- Demostración del ZEISS MICOR 700 aprobado por la FDA, el primer dispositivo de mano para la remoción de lentes con operación sin ultrasonido
- Expansión del ZEISS Retina Workflow para incluir soluciones quirúrgicas vitreorretinal avanzadas de DORC
- Aprobación de la FDA para los láseres terapéuticos de nueva generación ZEISS VISULAS combi y láseres verdes
Estas innovaciones tienen como objetivo avanzar en los flujos de trabajo clínicos y respaldar la atención personalizada en tratamientos de cataratas, refracción corneal, retina y glaucoma.
ZEISS 메디컬 테크놀로지는 2024년 10월 19일부터 21일까지 시카고에서 열리는 미국 안과학회(AAO) 회의에서 새로운 디지털 개선 및 수술 솔루션을 선보이고 있습니다. 주요 하이라이트는 다음과 같습니다:
- ZEISS VisioGen의 도입, 굴절 환자와의 소통을 향상시키고 클리닉 운영을 간소화하는 AI 기반 솔루션
- 1천만 개의 눈 치료 기념, ZEISS SMILE 및 ZEISS SMILE pro 렌티큘 추출 솔루션
- 25년의 리더십 기념, ZEISS IOLMaster 생체계 측정기
- FDA 승인 ZEISS MICOR 700 시연, 초음파 없는 작동으로 렌즈 제거가 가능한 최초의 핸드헬드 장치
- ZEISS Retina Workflow의 확장, DORC의 고급 유리체 망막 수술 솔루션 포함
- 차세대 ZEISS VISULAS combi 및 그린 치료 레이저에 대한 FDA 승인
이러한 혁신은 임상 워크플로우를 발전시키고 백내장, 각막 굴절, 망막 및 녹내장 치료에 대한 개인 맞춤 치료를 지원하는 것을 목표로 하고 있습니다.
ZEISS Technologie Médicale présente de nouvelles améliorations digitales et des solutions chirurgicales lors de la conférence de l'American Academy of Ophthalmology (AAO) qui se tiendra du 19 au 21 octobre 2024 à Chicago. Les points forts comprennent :
- Introduction de ZEISS VisioGen, une solution alimentée par IA pour améliorer la communication avec les patients réfractifs et rationaliser les opérations cliniques
- Célébration de 10 millions d'yeux traités avec les solutions d'extraction lenticulaire ZEISS SMILE et ZEISS SMILE pro
- Marquage de 25 ans de leadership en biométrie optique avec les biomètres ZEISS IOLMaster
- Démo du ZEISS MICOR 700 approuvé par la FDA, le premier dispositif portable d'enlèvement de lentilles fonctionnant sans ultrasons
- Expansion du ZEISS Retina Workflow pour inclure des solutions chirurgicales vitreorétinales avancées de DORC
- Approbation de la FDA pour la prochaine génération de ZEISS VISULAS combi et lasers thérapeutiques verts
Ces innovations visent à améliorer les flux de travail cliniques et à soutenir des soins personnalisés dans les traitements des cataractes, de la réfraction cornéenne, de la rétine et du glaucome.
ZEISS Medizintechnologie präsentiert neue digitale Verbesserungen und chirurgische Lösungen auf der Konferenz der American Academy of Ophthalmology (AAO) vom 19. bis 21. Oktober 2024 in Chicago. Zu den wichtigsten Höhepunkten gehören:
- Einführung von ZEISS VisioGen, einer KI-gesteuerten Lösung zur Verbesserung der refraktiven Patientenkommunikation und Optimierung der Klinikabläufe
- Feier von 10 Millionen behandelten Augen mit den ZEISS SMILE und ZEISS SMILE pro Lenticule-Extraktionslösungen
- Markierung von 25 Jahren Führung in der optischen Biometrie mit den ZEISS IOLMaster-Biomtern
- Demonstration des von der FDA zugelassenen ZEISS MICOR 700, dem ersten tragbaren Gerät zur Linsenentfernung ohne Ultraschallbetrieb
- Erweiterung des ZEISS Retina Workflow um fortschrittliche vitreoretinale chirurgische Lösungen von DORC
- FDA-Zulassung für die nächste Generation von ZEISS VISULAS combi und grünen therapeutischen Lasern
Diese Innovationen zielen darauf ab, klinische Arbeitsabläufe zu verbessern und personalisierte Pflege in der Behandlung von Katarakten, hornhautfreien Refraktionen, Netzhauterkrankungen und Glaukom zu unterstützen.
- Introduction of ZEISS VisioGen, an AI-driven solution to enhance patient communication and streamline clinic operations
- Over 10 million eyes treated with ZEISS SMILE and ZEISS SMILE pro lenticule extraction solutions
- 25 years of leadership in optical biometry with ZEISS IOLMaster becoming the most commonly used biometer
- FDA clearance and availability of ZEISS MICOR 700, the first hand-held lens removal device with ultrasound-free operation
- Expansion of ZEISS Retina Workflow to include advanced vitreoretinal surgical solutions from DORC
- FDA clearance for next-generation ZEISS VISULAS combi and green therapeutic lasers
- None.
Showcasing at AAO 2024:
- ZEISS VisioGen offers a cutting-edge, AI-driven solution designed to enhance refractive patient communication and streamline clinic operations.
- ZEISS Milestone: Celebrating more than 10 million eyes treated with lenticule extraction solutions utilizing ZEISS SMILE and ZEISS SMILE pro.
- ZEISS marked its 25th year of leadership in defining optical biometry, with ZEISS IOLMaster biometers becoming the most commonly used biometers in the ophthalmic world.
- ZEISS MICOR 700 puts the future of lens extraction in the hands of surgeons as the first hand-held lens removal device with ultrasound-free operation; the FDA-cleared device is available in the
U.S. - Expanding the ZEISS Retina Workflow, now to include advanced vitreoretinal surgical solutions from DORC.
- Next-generation ZEISS VISULAS combi and green therapeutic lasers receive FDA clearance.
JENA,
"ZEISS continues to expand its digital leadership in ophthalmology, offering new, pioneering ophthalmic offerings and clinical tools that create an enhanced digital workflow experience for both patients and surgeons," said Euan S. Thomson, Ph.D., Head of the Digital Business Unit for ZEISS Medical Technology. "With the foundation of our Health Data Platform as part of the ZEISS Medical Ecosystem, our data-driven healthcare solutions unlock enormous value for surgeons, helping them deliver more efficient and personalized care throughout a patient's journey."
ZEISS transforms the Refractive Workflow from patient engagement to enhanced efficiency
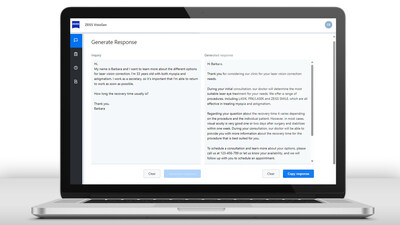
Extending the ZEISS digital portfolio, the company will introduce ZEISS VisioGen, a cutting-edge AI-driven solution designed to enhance refractive patient communication and streamline clinic operations, expanding the value to ophthalmologists and clinics by helping them grow their business through improved patient engagement. ZEISS VisioGen leverages generative AI (GenAI) for effective and efficient patient acquisition-as-a-service. The software solution provides digital communication services to offer fast, high-quality responses to patients. ZEISS VisioGen enables clinics to respond more effectively to patient inquiries while converting more patients to consultations using the latest GenAI technology combined with substantiated and verified content from ZEISS to generate personalized draft responses to patient queries for clinical staff to finalize and use.
"ZEISS VisioGen is an extremely useful tool for the busy refractive practice. It can save time and prevent grammatical errors in responding to questions from potential patients. The response time is quick and useful. Responses can also be effectively tailored to the specifics of your individual practice," said Dr. Luke Rebenitsch, MD, ClearSight,
Demonstrating its continued momentum in the LVC market, ZEISS recently celebrated that more than 10 million eyes have been treated with lenticule extraction solutions utilizing ZEISS SMILE and ZEISS SMILE pro, marking a significant milestone for the company and proof of the growing international adoption of safe and effective lenticule extraction solutions. In 2011, ZEISS was the first medical device manufacturer to make lenticule extraction for laser vision correction commercially available, and ZEISS SMILE and ZEISS SMILE pro continue to be leading1 solutions trusted by surgeons for the technology's reliability and effective treatment history with the VisuMax® and VISUMAX® 800 from ZEISS.
ZEISS will also showcase the recently FDA-approved VISUMAX® 800 with SMILE® pro software from ZEISS for surgically treating nearsightedness, with or without astigmatism. The updated ZEISS femtosecond laser provides
ZEISS continues to reshape cataract surgery with revolutionary innovation
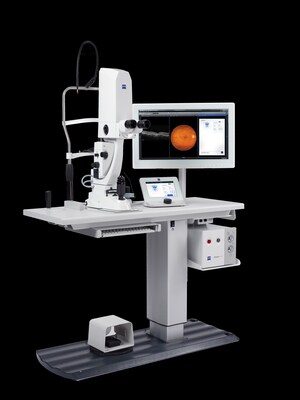
With a long heritage in optics, ZEISS has been shaping optical biometry for 25 years since the introduction of the first automated non-invasive optical biometer. Marking a significant milestone in cataract surgery, the ZEISS IOLMaster, was the first device to combine contactless keratometry, axial length measurement, and IOL calculation. This innovation opened the door for continuous advancements in biometry, setting a new standard for getting fewer refractive surprises. Today, the ZEISS IOLMaster is the most commonly used biometer in the ophthalmic world.
Recently FDA cleared and now broadly available in the
ZEISS will also showcase its expanded portfolio of high-end microscopes with the ZEISS ARTEVO® 850 3D heads-up ophthalmic microscope setting the pace in digital visualization with true color imaging, and increased depth of field by nearly 60 percent.3 Additionally, the ZEISS ARTEVO 850 with CALLISTO eye® features a redesigned intuitive user interface that centralizes all controls on a single touchscreen. The second latest addition to ZEISS's portfolio of optical surgical microscopes, the new ARTEVO® 750, elevates the surgical workflow by introducing advanced optical visualization technology, including new RGB LED illumination with adjustable light color temperature, as well as data overlays provided in the eyepiece with a 40 percent increase in resolution.4 The two devices are CE-marked and FDA cleared and commercially available in all major markets.
ZEISS redefines the future of retinal care, optimizing disease detection, management and treatment
ZEISS is redefining the future of retinal care, offering a holistic approach that empowers specialists to deliver excellent patient care throughout the entire patient journey. At AAO, ZEISS will showcase its comprehensive portfolio across the entire ZEISS Retina Workflow, now combining cutting-edge diagnostic solutions with advanced visualization and vitreoretinal surgical systems and next generation therapeutic lasers. The ZEISS Retina Workflow offers connected and integrated solutions that help eyecare professionals to deliver optimized patient care from early detection, through monitoring, to in-office or surgical treatments.
ZEISS will showcase cutting-edge imaging technology and 3D visualization as part of the ZEISS Retina Workflow. The CIRRUS® 6000 from ZEISS introduces an expanded Reference Database, triple that of its previous database, with greater diversity and three different disc sizes for more individualized patient care. With an acquisition speed of 100,000 A scans per second, the device provides instantaneous dense data cubes and sub-10-second-wide-field OCT Angiography capability, together with automated 9 slab standard report presentation to complement the review process. Setting the pace in digital visualization, the ZEISS ARTEVO 850 includes customizable digital color settings depending on the surgical procedure's needs and intraoperative OCT allowing for real-time monitoring of the surgical process and decision-making.
The newest addition to the ZEISS Retina Workflow, the DORC EVA NEXUS™ surgical system and instrumentation bring added value and synergy to the OR. The DORC vitreoretinal portfolio includes the EVA NEXUS™ phacovitrectomy system featuring the unique VTi pump, offering FLOW and VACUUM fluidics, EVA AVETA™ trocar cannula system with Push-Fit HI-FLOW™ infusion connection, TDC (two-dimensional cutting) vitrectomy with cut speeds of up to 20,000 CPM 5, EVA INICIO™ microinjection system, and an extensive range of posterior instruments including 27G ULTRA for no-compromise, small-gauge surgery, as well as a wide range of highly purified posterior surgical liquids and tamponades.
In addition, the next generation therapeutic laser portfolio received 510k FDA clearance for two models, the VISULAS® combi, which combines YAG and 532 modalities, and the standalone VISULAS® green (532) laser. One of the key benefits of the ZEISS VISULAS portfolio is the digital connection to the FORUM® data management solution, allowing surgeons to seamlessly integrate laser therapy into the ZEISS Retina Workflow, ensuring a high level of efficiency throughout the entire process.
ZEISS will showcase its latest offerings and new innovations at the American Academy of Ophthalmology (AAO) conference from Oct. 19-21, 2024, in
1 | Market Scope Refractive Surgery Report 2023, Global Refractive Market (Manufacturer Level) incl RLE | Refractive Market by Estimated Total Revenue, p. 273. | |
2 | Data on file, myopia with optical zone 6.5 mm. | |
3 | Compared to ZEISS ARTEVO 800. | |
4 | Compared to previous generation ZEISS LUMERA 700. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems. | |
5 | 20,000 refers to the cuts per minute, the EVA NEXUS™ outputs 10,000 cycles per minute. | |
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support. | ||
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704) is one of the world's leading medical technology companies and is included in the German MDAX and TecDAX stock indices. The company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The company offers complete solutions for the diagnosis and treatment of eye diseases – including implants and consumables. In the field of microsurgery, the company provides innovative visualization solutions. With 4,224 employees worldwide, the company generated revenue totaling
The company is headquartered in Jena,
For further information visit: www.zeiss.com/med
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-expands-ophthalmic-offerings-to-improve-patient-care-with-new-digital-ai-tools-and-revolutionary-surgical-solutions-302271146.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/zeiss-expands-ophthalmic-offerings-to-improve-patient-care-with-new-digital-ai-tools-and-revolutionary-surgical-solutions-302271146.html
SOURCE Carl Zeiss Meditec AG